Affinity chromatography is a specialized chromatographic technique used in biochemistry and molecular biology to separate and purify biomolecules such as proteins, nucleic acids, and other macromolecules based on their particular interactions with a ligand or affinity matrix. It is a method of extracting a biomolecule from a mixture based on a highly precise macromolecular binding interaction between the biomolecule and another material.
The extremely selective and reversible binding between a target molecule (the analyte) and a particular ligand immobilized on a solid substrate (the matrix) is used in this approach. The type of binding relationship used depends on the biomolecule of interest; antigen and antibody, enzyme and substrate, receptor and ligand, or protein and nucleic acid binding interactions are all commonly used for biomolecule isolation. When compared to other chromatographic procedures, it is helpful for its excellent selectivity and separation resolution.
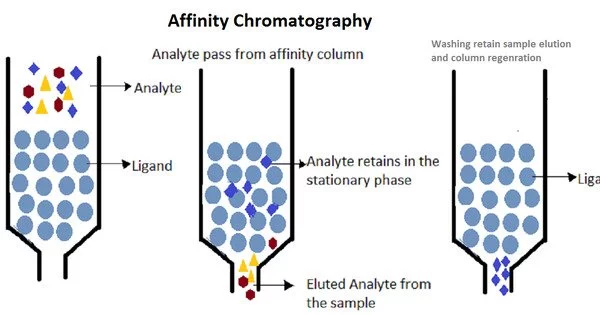
How affinity chromatography works:
- Ligand Immobilization: A ligand is covalently linked to a chromatography matrix. A ligand is a chemical that can selectively interact with the target biomolecule of interest. Antibodies, antigens, enzymes, receptor ligands, and other molecules with a high affinity for the target are examples of common ligands.
- Sample Application: The sample containing the biomolecule combination is applied to the column containing the immobilized ligand.
- Binding: Non-specific molecules pass through or bind poorly to the ligand on the matrix, whereas the target biomolecule binds selectively.
- Washing: Following sample loading, the column is routinely washed with a buffer to eliminate any weakly bound or loose molecules, resulting in high specificity in the purification process.
- Elution: The specifically bound target molecule can be eluted by altering the conditions, such as changing the pH or ionic strength of the buffer or adding a competing ligand. This disrupts the binding interaction and releases the target molecule from the column.
Because of its great specificity and selectivity, affinity chromatography is beneficial. It can identify and purify particular proteins, antibodies, enzymes, and other biomolecules from complex mixtures while removing undesirable contaminants. This technique is widely employed in biotechnology disciplines such as medicines, biopharmaceuticals, and basic research.
Affinity chromatography is divided into several types, including immobilized metal ion affinity chromatography (IMAC), protein A/G chromatography, and lectin affinity chromatography, each of which is designed to exploit particular interactions between ligands and their target molecules. The proper ligand and circumstances are determined by the nature of the biomolecule being purified.