Remodeling the immune system to improve its ability to combat tuberculosis (TB) is an ongoing research topic, with numerous ways being considered. Tuberculosis, caused by the bacteria Mycobacterium tuberculosis (Mtb), kills up to 1.6 million people each year, making it one of the top causes of mortality from an infectious agent worldwide – and the number is only increasing.
The exact mechanism by which Mtb evades the immune system is unknown, but a collaborative team of researchers from the University of Massachusetts Amherst and Seattle Children’s Research Institute recently discovered something surprising: prior exposure to the Mycobacterium genus appears to remodel the body’s first-line defenders. Furthermore, how those cells are altered is determined by the specific conditions in which the body is exposed. These findings, published recently in PLOS Pathogens, indicate that a more integrated therapy approach that addresses all parts of the immune response may be a more effective strategy in the fight against tuberculosis.
“We breathe in thousands of liters of air every day,” says Alissa Rothchild, assistant professor in the Veterinary and Animal Sciences Department at UMass Amherst and the paper’s senior author. “This essential process makes us incredibly vulnerable to inhalation of all sorts of potentially infectious pathogens that our immune systems have to respond to.”
We breathe in thousands of liters of air every day. This essential process makes us incredibly vulnerable to inhalation of all sorts of potentially infectious pathogens that our immune systems have to respond to.
Alissa Rothchild
Systems, plural. When we think of immunity, we typically think of the adaptive immune system, which is when prior exposure to a pathogen – say, a weakened version of chickenpox — teaches the immune system what to guard against. Vaccination is the most common tool that we use to teach our adaptive immune systems what to look out for.
While the adaptive immune system is the major focus of most vaccine research (think protective antibodies induced by COVID-19 vaccines), it is not the body’s first responder – that would be the innate immune system and its ranks of macrophages. The macrophages are the first-line defenders in the tissues that recognize and destroy pathogens and also call for backup. One way they do this by turning on different inflammatory programs that can change the tissue environment.
In the case of the lungs, these macrophages are called alveolar macrophages (AMs). They live in the lung’s alveoli, the tiny air sacs where oxygen passes into the bloodstream — but, as Rothchild has shown in a previous paper, AMs don’t mount a robust immune response when they’re initially infected by Mtb. This lack of response seems to be a chink in the body’s armor that Mtb exploits to such devastating effect. “Mtb takes advantage of the immune response,” says Rothchild, “and when they infect an AM, they can replicate inside of it for a week or longer. They effectively turn the AM into a Trojan Horse in which the bacteria can hide from the body’s defenses.”
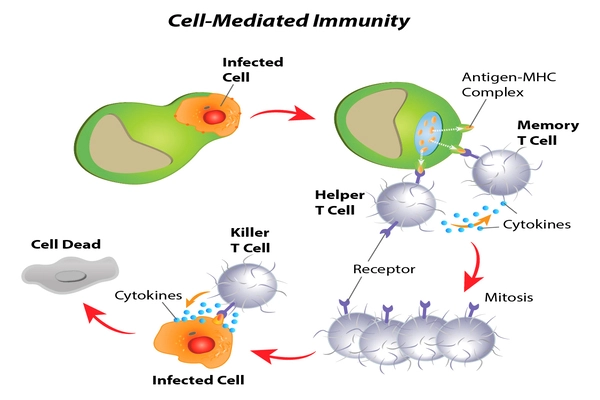
“But what if we could change this first step in the chain of infection?” Rothchild continues. “What if the AMs responded more effectively to Mtb? How could we change the body’s innate immune response? Studies over the last 10 years or so have demonstrated that the innate immune system is capable of undergoing long-term changes, but we are only beginning to understand the underlying mechanisms behind them.”
To investigate situations in which the innate immune response could be altered, Dat Mai, a research associate at Seattle Children’s Research Institute, and the paper’s first author, Rothchild, and their colleagues designed an experiment with two different mouse models. The initial model made use of the BCG vaccine, which is one of the world’s most extensively circulated vaccinations and the only one used to treat tuberculosis. In the second model, the researchers established a confined Mtb infection, which they had previously demonstrated to protect against subsequent infections through concurrent immunity.
Weeks after exposure, the researchers challenged the animals with aerosolized Mtb, and infected macrophages from each mouse model were collected for RNA sequencing. There were significant changes in the RNA between the models.
While both sets of AMs demonstrated a stronger pro-inflammatory response to Mtb than AMs from unexposed mice, the BCG-vaccinated AMs strongly activated one type of inflammatory program, driven by interferons, whereas the AMs from the contained Mtb infection activated a qualitatively different inflammatory program. Other investigations revealed that the various exposure circumstances altered the AMs themselves, with some of these modifications appearing to be dependent on the larger lung environment.
“What this tells us,” Rothchild says, “is that there’s a great deal of plasticity in the macrophage response, and that there’s potential to therapeutically harness this plasticity so that we can remodel the innate immune system to fight tuberculosis.”