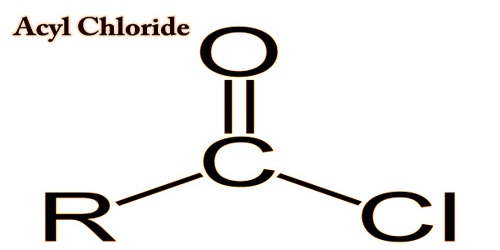
An acyl chloride, also known as acid chloride in organic chemistry, is an organic compound consisting of a chlorine atom bound to an acyl ring. Typically their formulation is written RCOCl, where R is a side chain. There are a number of related compounds which replace the -OH group in the acid with something else. Such compounds are described as acid-derivatives. A specific example of an acyl chloride is acetyl chloride, CH3COCl. The most critical subset of acyl halides are the acyl chlorides.
An acyl chloride similar to chloride from ethanoyl is a colorless fuming liquid. Ethanoyl chloride’s strong smell is a mixture of vinegar odor (ethanoic acid) and acrid hydrogen chloride gas odour. Functional groups are parts of a molecule which is understood to be a recognized bonded atom group. Where the acyl chloride moiety takes priority, acyl chlorides are named by taking the name of the parent carboxylic acid and substituting -yl chloride for -ic acid. Thus:
acetyl chloride CH3COCl
benzoyl chloride C6H5COCl
When other functional groups take priority, acyl chlorides are considered prefixes chlorocarbonyl: (chlorocarbonyl) acetic acid ClOCCH2COOH
An acid chloride’s aversion to forming hydrogen bonds with other compounds is a property of. The scent and the gases are due to the interaction of ethanoyl chloride in the air with water vapour. Acetic acid, for example, boils at 118 ° C, while acetyl chloride boils at 51 ° C. Infrarot spectroscopy, like most carbonyl compounds, shows a band close to 1750 cm−1.
A hydrogen bond is a chemical bond created by attracting a hydrogen atom, in one molecule, to an electronegative atom on another molecule. Electronegative, refers simply to an atom that likes to attract electrons to form a chemical bond. Ethanoyl chloride or acetyl chloride is the most stable acyl chloride; methanoyl chloride (formyl chloride) is not stable at room temperature, but it may be prepared at or below -60 ° C. Acyl chloride doesn’t get water-soluble. Then it breaks down into the mud.
Acyl chlorides cannot be said to dissolve in water, as they react with it (often violently). The strong reaction means a simple, aqueous solution of an acyl chloride cannot be obtained. Acyl chlorides are usually prepared in the laboratory in the same way as alkyl chlorides, by replacing the corresponding hydroxy substituents with chlorides. Acyl chlorides are highly reactive, and the chlorine atom is replaced by other things in their reactions. To give primary alcohols, acyl chlorides are reduced with lithium aluminum hydride and diisobutylaluminum hydride.
Information Sources: