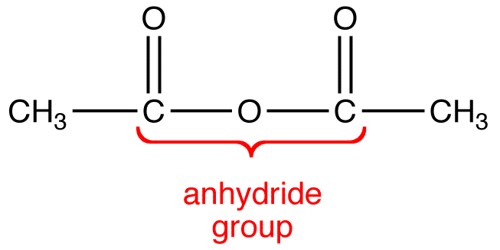
Acid Anhydride is a compound with two acyl groups bound to a single oxygen atom. It is the product of formal condensation of two oxoacid molecules with the release of a water molecule. It is a nonmetal oxide which reacts with water to form an acidic solution.
- Acids – These are the substances that are ready to donate hydrogen ions in water.
- Bases – these are the substances that hydroxide ions in water.
An acid anhydride is a type of chemical compound derived by the removal of water molecules from an acid. It is a compound from which water has been abstracted. They formed by the loss of water between two carboxyl groups, can survive long enough in aqueous solutions to react with protein amines. Acid anhydride also refers to compounds containing the acid anhydride functional group. They are used as plasticizers for PVC and other plastics, especially where temperature stability is required, as for example in coatings for wires and cables.
In organic chemistry, organic acid anhydrides contain the functional group R(CO)O(CO)R’. The most common anhydrides in organic chemistry are those derived from carboxylic acids. Organic acid anhydrides often form when one equivalent of water is removed from two equivalents of an organic acid in a dehydration reaction. They are used in a variety of chemical processes but particularly in epoxy resins.
In inorganic chemistry, an acid anhydride refers to an acidic oxide, an oxide that reacts with water to form an oxyacid (an inorganic acid that contains oxygen or carbonic acid), or with a base to form a salt. Carboxylic acid anhydrides have the general structural formula 1, in which R1 and R2 could be hydrogen atoms, alkyl groups, aryl groups, or any combination thereof. Acid anhydrides are named from the acids that created them. The “acid” part of the name is replaced with “anhydride.” For example, the acid anhydride formed from acetic acid would be acetic anhydride. They have direct irritant effects and also can sensitize individuals, leading to occupational asthma. So we can conclude that all acid anhydrides are non-metals and all non-metals are not acid anhydride. Anhydrides of organic acids, like the acids themselves, contain the carbonyl group, CO. For example, carbon monoxide is not an acid anhydride, though it is an oxide of carbon because it does not react with water.