Fuel cell-powered heavy-duty hydrogen vehicles must have more durable and efficient fuel cells in order to compete with their combustion-fuelled rivals. A novel approach to studying and comprehending the degradation of fuel cell components over time has been devised by researchers at Chalmers University of Technology in Sweden. This is a significant step toward fuel cells’ enhanced performance and eventual commercial success.
For heavy-duty vehicles, hydrogen is an increasingly appealing fuel substitute. Vehicles that run on hydrogen only release water vapor as exhaust, and if the hydrogen is generated using renewable energy, no carbon dioxide is released at all. Since hydrogen can be created and stored when electricity is cheap, hydrogen-powered electric vehicles do not need to strain the electrical grid like battery-powered ones do.
For some hydrogen-powered vehicles the propulsion comes from a so-called fuel cell. However, hydrogen-fuel-cell-powered vehicles are limited by a relatively short lifespan, because fuel cell components, such as electrodes and membranes, degrade over time. It is this problem that the recent study addresses.
It has previously been assumed that the performance would be affected by the fuel cell being disassembled and studied in the way we have done, but it turned out that this assumption is not correct, which is surprising.
Björn Wickman
Researchers at Chalmers University of Technology have developed a new method for studying what affects the ageing of fuel cells by tracking a specific particle in the fuel cell during use. The team of researchers have studied an entire fuel cell by taking it apart at regular intervals. Using advanced electron microscopes, they have then been able to follow how the cathode electrode degrades in specific areas during the cycles of use. Previous studies have been done on so-called half-cells, which are similar (but not the same as) half of a fuel-cell and are carried out under conditions that differ significantly from the real fuel cell.
Better understanding with new experimental method
“It has previously been assumed that the performance would be affected by the fuel cell being disassembled and studied in the way we have done, but it turned out that this assumption is not correct, which is surprising,” says research leader Björn Wickman, Associate Professor at the Department of Physics at Chalmers.
The researchers at Chalmers have been able to explore how the material in the fuel cell degrades at both the nano and micro level, and pinpoint exactly when and where the degradation occurs. This provides valuable information for the development of new and improved fuel cells with a longer lifespan.
“From previously only looking at how the fuel cell has aged after use, we have now been able to look into the middle stage,” says doctoral student Linnéa Strandberg at Chalmers. “Being able to follow a single, chosen particle within a specific area, provided a much better understanding of the degradation processes. Greater knowledge of these is an important step on the way to designing new materials for fuel cells or to adjust the control of the fuel cell.”
New method paves way for longer lasting fuel cells
The U.S. Department of Energy (DOE) has pointed out that improved lifetime of fuel cells is one of the most important goals to reach before fuel cell-powered hydrogen vehicles can become commercially successful. According to the industry, a truck needs to be able to withstand 20,000 — 30,000 hours of driving over its lifetime, which a fuel cell-powered hydrogen truck cannot achieve today.
“We have now laid a foundation on which to build for the development of better fuel cells. Now we know more about the processes that take place in the fuel cell and at what point over the lifetime of the fuel cell they occur. In the future, the method will be used to develop and study new materials that can give the fuel cell a longer lifespan,” says Björn Wickman.
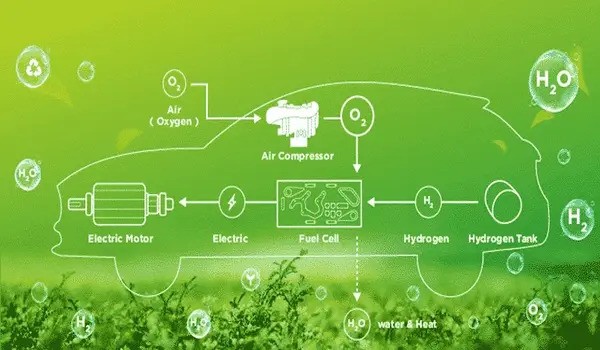
Facts: How a fuel cell works
The core of a fuel cell consists of three active layers, two electrodes — anode and cathode respectively — with an ion-conducting membrane in the middle. Each individual cell provides a voltage of about 1 volt. The electrodes contain catalyst material, and hydrogen and oxygen are added to them. The resulting electrochemical process generates clean water and electricity that can be used to power a vehicle.
More about the research:
The research group at Chalmers that has developed the method consists of doctoral student Linnéa Strandberg and Associate Professor Björn Wickman, both at the Department of Physics, Victor Shokhen, former postdoc at the Department of Physics, and Magnus Skoglundh, professor at the Department of Chemistry and Chemical Engineering.