Researchers are constantly attempting to create more efficient and cost-effective catalysts for the electrochemical reactions that occur during water splitting. A unique approach to water electrolysis catalysts for green hydrogen production has been developed by scientists.
Originally, the term “Sherpa” referred to a Tibetan hill tribe, but it has since become synonymous with guides on Mount Everest, the world’s tallest and most treacherous mountain. Research into the difficult process of building catalysts for hydrogen production, much like these Sherpas, is making significant progress and has garnered recognition as the featured cover article in an international publication.
Professor Yong-Tae Kim of the Department of Materials Science and Engineering and the Graduate Institute of Ferrous & Eco Materials Technology at Pohang University of Science and Technology (POSTECH) and Kyu-Su Kim, a doctoral student from the Department of Materials Science and Engineering, collaborated on a research project that offers a promising direction for the future development of catalysts for water electrolysis. Their research has received a lot of attention from academics, and it was featured as the cover article in ACS Catalysis, an international magazine in the field of chemistry.
This research marks the beginning of our journey, not the end. We are dedicated to the continuous development of efficient water electrolysis catalysts based on the insights gained from this research.
Professor Yong-Tae Kim
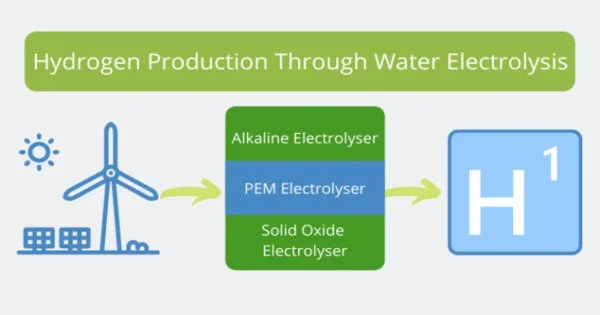
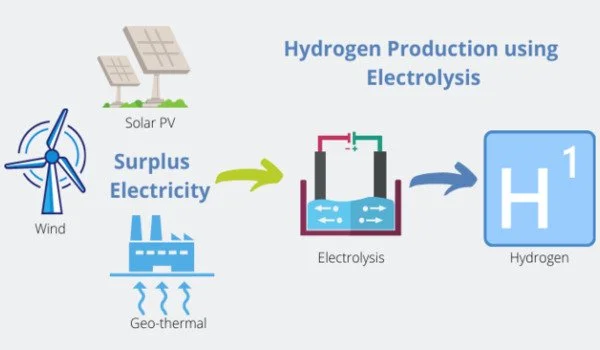
Water electrolysis, a method of creating hydrogen from the vast resource of water, appears as an environmentally benign technology that emits no CO2. However, because of its dependency on precious metal catalysts such as iridium (Ir), this approach is commercially unfeasible. To overcome this difficulty, researchers are currently investigating the development of catalysts in the form of metal alloys.
The principal catalysts under investigation in the field of water electrolysis catalysis research are iridium, ruthenium (Ru), and osmium (Os). Despite its excellent stability, iridium has minimal activity and a high price. Ruthenium, on the other hand, has commendable activity and is a more cost-effective alternative than iridium, however, it lacks the same level of stability. Osmium, on the other hand, quickly dissolves under a variety of electrochemical circumstances, resulting in the creation of nanostructures with increased electrochemical active surface area and geometrical activity.
Initially, the research team developed catalysts using both iridium and ruthenium. By combining these metals, they successfully preserved the excellent attributes of each, resulting in catalysts that demonstrated improvements in both activity and stability. Catalysts incorporating osmium exhibited high activity due to the expanded electrochemical active surface area achieved through nanostructure formation. These catalysts retained the advantageous properties of iridium and ruthenium.
As a result, the researchers broadened their research to cover all three metals. The results revealed a small increase in activity, but osmium dissolution had a negative impact, considerably jeopardizing the structural integrity of iridium and ruthenium. The aggregation and corrosion of nanostructures were increased in this series, resulting in a decrease in the balance of catalytic performance.
The research team has identified various possibilities for further catalyst research based on these findings. First and foremost, they emphasize the importance of a metric that can assess both activity and stability at the same time. Kim’s research group first introduced this metric, known as the activity-stability factor, in an international journal in 2017.
Furthermore, the team pushes for the retention of outstanding catalytic capabilities even after nanostructure development in order to increase the electrochemically active surface area of the electrocatalyst. They also emphasize the significance of carefully selecting candidate materials that can effectively synergize with other metals when alloyed. The purpose of this study is not to give specific outcomes such as the development of new catalysts, but rather to provide basic principles for catalyst design.
Professor Yong-Tae Kim, who led the study, stated, “This research marks the beginning of our journey, not the end.” He furthers on his goal by saying, “We are dedicated to the continuous development of efficient water electrolysis catalysts based on the insights gained from this research.”