Cellulose is an important component of plant cell walls, providing structural support and rigidity. If a previously unknown protein is discovered to regulate cellulose production in plant cells, it could have far-reaching implications for our understanding of plant growth and development.
A team has discovered a protein that modifies the cellular machinery responsible for cellulose production, which could help in the development of more stable, cellulose-enriched materials for biofuels and other applications.
Cellulose, an essential component of plant cell walls, is a valuable source of food, paper, textiles, and biofuels, but how it is produced within plant cells is unknown. A team led by Penn State researchers has now identified a protein that modifies the cellular machinery responsible for cellulose production, ultimately lending stability to that machinery. This new understanding could help to design more stable, cellulose-enriched materials for biofuels and other applications.
A complex of proteins known as the cellulose synthase complex constructs a chain of cellulose within a plant cell. The regulation of this process determines a number of properties, including when and how quickly it occurs, as well as the length of the cellulose chain.
Cellulose is the most abundant biopolymer on Earth, yet despite its importance, relatively little is known about how its synthesis is regulated. In this study, we identified a protein called calcium-dependent protein kinase 32 (CPK32) and confirmed that it chemically modifies one of the proteins in the cellulose synthase complex, ultimately helping to regulate the cellulose biosynthesis process.
Ying Gu
“Cellulose is the most abundant biopolymer on Earth, yet despite its importance, relatively little is known about how its synthesis is regulated,” said Ying Gu, professor of biochemistry and molecular biology in the Penn State Eberly College of Science and leader of the research team. “In this study, we identified a protein called calcium-dependent protein kinase 32 (CPK32) and confirmed that it chemically modifies one of the proteins in the cellulose synthase complex, ultimately helping to regulate the cellulose biosynthesis process.”
The researchers published their findings in the journal New Phytologist.
The CPK32 protein’s chemical modification is known as phosphorylation; it adds a chemical compound known as a phosphor group to the cellulose synthase protein CESA3. These types of modifications are reversible and help the cell perform a variety of important biological functions. More than 200,000 locations on proteins in humans can be phosphorylated by more than 500 proteins known as kinases. More than 43,000 locations in the plant Arabidopsis, also known as thale cress and widely used in plant science, can be phosphorylated by more than 1,000 kinases.
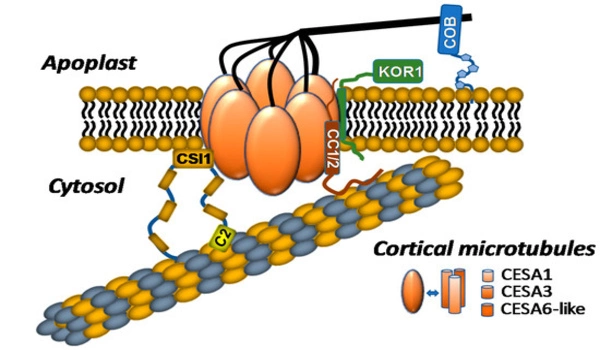
“Identifying which of the many kinases could phosphorylate cellulose synthase was very daunting,” said Gu. “We used a screening approach to look for proteins that directly associate with CESA3. This revealed the kinase CPK32, and we followed up with a series of experiments to confirm that CPK32 actually phosphorylates CESA3, to identify the specific location on CESA3 where this occurs, and to determine how this phosphorylation impacts the plant.”
The researchers then engineered a CESA3 protein with a mutation that changed the site where the phosphor group is added, preventing phosphorylation. Cells from mutated plants with no phosphorylation of CESA3 had lower cellulose content and lower cellulose synthase complex stability, and adult plants from mutated plants had stunted growth.
“Previous studies have shown that CPK32 plays a role in several biological processes, including pollen tube growth as well as shoot and root development,” Gu explained. “We show here that CPK32 has a new function and a novel phosphorylation mechanism in stabilising the cellulose synthase complex.”
The researchers intend to investigate whether CESA3 phosphorylation is unique to CPK32 or if other kinases in the same family can regulate cellulose biosynthesis in the same way.
“By regulating the stability of the cellulose synthase complex, we may be able to encourage cells to produce longer cellulose chains and, ultimately, engineer cellulose-rich materials,” Gu explained.