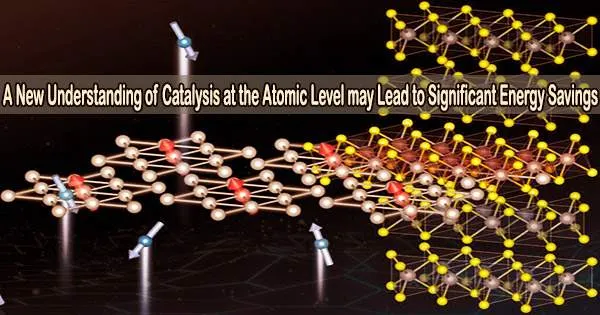
Chemical engineers at the University of Wisconsin-Madison have created a model of how catalytic reactions operate at the atomic level, a development they regard to be a breakthrough in computational chemistry research.
Given that 90% of the items we use in our daily lives are created, at least in part, through catalysis, this insight could help engineers and chemists create more effective catalysts and fine-tune industrial processes with the potential to save significant amounts of energy.
Materials that act as catalysts quicken chemical reactions without changing themselves. In addition to producing medications, polymers, food additives, fertilizers, green fuels, industrial chemicals, and much more, they are essential for the refinement of petroleum products.
Scientists and engineers have spent decades perfecting catalytic reactions, but they haven’t fully understood what is happening on the nano and atomic scales because it’s currently not possible to directly observe those reactions at the extreme temperatures and pressures frequently involved in industrial-scale catalysis. This new study contributes to the solution of that puzzle and may have significant implications for business.
In actuality, only three catalytic reactions methane reforming to produce hydrogen, ammonia synthesis to create fertilizer, and methanol synthesis consume close to 10% of the energy in the world.
“If you decrease the temperatures at which you have to run these reactions by only a few degrees, there will be an enormous decrease in the energy demand that we face as humanity today,” says Manos Mavrikakis, a professor of chemical and biological engineering at UW-Madison who led the research. “By decreasing the energy needs to run all these processes, you are also decreasing their environmental footprint.”
Mavrikakis and postdoctoral researchers Lang Xu and Konstantinos G. Papanikolaou along with graduate student Lisa Je published news of their advance in the April 7, 2023 issue of the journal Science.
The engineers at UW-Madison create and apply potent modeling techniques to simulate catalytic reactions at the atomic scale in their study. In this work, transition metal catalysts in nanoparticle form which include metals like platinum, palladium, rhodium, copper, nickel, and others crucial to industry and renewable energy were examined in reactions utilizing these catalysts.
The closely packed atoms of transition metal catalysts form a 2D surface that chemical reactants adhere to and engage in reactions, according to the current rigid-surface model of catalysis. The bonds between the atoms in the chemical reactants are broken when enough pressure, heat, or electricity is applied, allowing the fragments to recombine into new chemical products.
“The prevailing assumption is that these metal atoms are strongly bonded to each other and simply provide ‘landing spots’ for reactants. What everybody has assumed is that metal-metal bonds remain intact during the reactions they catalyze,” says Mavrikakis. “So here, for the first time, we asked the question, ‘Could the energy to break bonds in reactants be of similar amounts to the energy needed to disrupt bonds within the catalyst?’”
According to Mavrikakis’s modeling, the answer is yes. Breaking bonds and allowing single metal atoms (sometimes referred to as “adatoms”) to break free and begin moving on the surface of the catalyst are both made possible by the energy required for many catalytic reactions to occur. These adatoms join together to form clusters, which act as catalyst sites where chemical reactions can occur far more easily than on the catalyst’s original stiff surface.
The team examined eight transition metal catalysts and 18 reactants in industrially significant interactions, using a set of specialized calculations to determine the energy levels and temperatures likely to form such small metal clusters as well as the number of atoms in each cluster, which can also significantly affect reaction rates.
Their experimental collaborators at the University of California, Berkeley, used atomically-resolved scanning tunneling microscopy to look at carbon monoxide adsorption on nickel (111), a stable, crystalline form of nickel useful in catalysis. Their research supported models that suggested different catalyst structural flaws could also affect how reaction sites occur and how individual metal atoms pop loose.
Mavrikakis says the new framework is challenging the foundation of how researchers understand catalysis and how it takes place. He will look into other non-metal catalysts in his upcoming study because it might also apply to them. It is pertinent to comprehending other significant processes as well, such as corrosion and tribology, or the interaction of moving surfaces.
“We’re revisiting some very well-established assumptions in understanding how catalysts work and, more generally, how molecules interact with solids,” Mavrikakis says.
Manos Mavrikakis is Ernest Micek Distinguished Chair, James A. Dumesic Professor, and Vilas Distinguished Achievement Professor in Chemical and Biological Engineering at the University of Wisconsin-Madison. Other authors include Barbara A.J. Lechner of the Technical University of Munich, and Gabor A. Somorjai and Miquel Salmeron of Lawrence Berkeley National Laboratory and the University of California, Berkeley.