Genome editing, also known as gene editing, is a field of study that aims to modify the genes of living organisms in order to better understand gene function and develop methods to use it to treat genetic or acquired diseases. A multidisciplinary team of researchers has developed techniques to boost the efficiency of CRISPR-Cas9, the genome editing technique that won the Nobel Prize in 2020.
A team of interdisciplinary researchers led by Penn State has developed techniques to improve the efficiency of CRISPR-Cas9, the genome editing technique that won the Nobel Prize in 2020. According to project leader Xiaojun “Lance” Lian, associate professor of biomedical engineering and biology at Penn State, while CRISPR-Cas9 is faster, less expensive, and more accurate than other gene-editing methods, the technology has limitations, particularly in applications to improve human health.
The researchers developed a more efficient and accessible method for using CRISPR-Cas9 systems in human pluripotent stem cells (hPSCs) derived from federally approved stem cell lines, which Lian believes could greatly advance genetic disorder diagnostics and treatments. Cell Reports Methods published the approach.
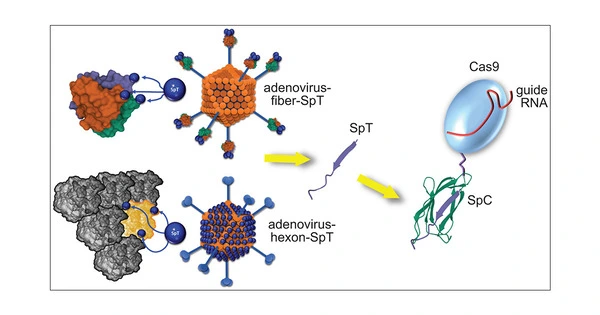
CRISPR-Cas9, which stands for clustered regularly interspaced short palindromic repeats and CRISPR-associated protein 9, gives scientists the ability to target precise locations of genetic code to change DNA, providing opportunities to create new diagnostic tools and potentially correct mutations to treat genetic causes of disease.
Delivery of DNA CRISPR effectors is low. When using CRISPR, only 20% to 30% of the targeted cells will receive gene-editing DNA; however, when regular RNA is introduced, cells may mistake it for a virus. They destroy the RNA before it can make proteins say, in a matter of a few hours and, in doing so, destroy the gene editing attempt.
Xiaojun Lian
“The human genome is enormous, and CRISPR-Cas9 makes it possible for scientists to find and target a mutated gene for the purpose of studying it,” Lian said.
CRISPR employs a disc of genetic material known as plasmid DNA to deliver guided ribonucleic acid (RNA) that positions the Cas9 enzyme at the precise location of the target gene. When the DNA is found, Cas9 binds to it and cuts it out, allowing other DNA to repair the cut. The researchers can then observe how the gene’s expression changes as a result of the removal. According to Lian, current DNA-based CRISPR methods have delivery and editing efficiency issues.
“Delivery of DNA CRISPR effectors is low,” he said. “When using CRISPR, only 20% to 30% of the targeted cells will receive gene-editing DNA; however, when regular RNA is introduced, cells may mistake it for a virus. They destroy the RNA before it can make proteins say, in a matter of a few hours and, in doing so, destroy the gene editing attempt.”
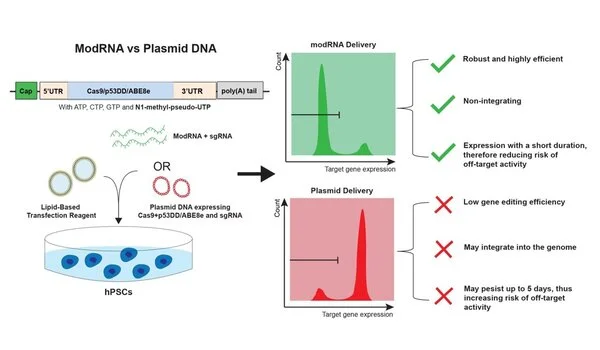
To improve the outcome, the researchers changed the way the genome editing tools are delivered to the stem cells, using modified RNA (modRNA). The modRNA differs from plasmid DNA in that it replaces one of the base substrates found in RNA with a chemically modified version, and it is stabilized by stronger structural support.
“The modRNA was found to be notably more efficient than plasmid DNA,” Lian said. “Around 90% of the cells received the modRNA from a simple transfection, so it was able to remain in place and do its job.”
The researchers also discovered that the duration of the modRNA was ideal: long enough to modify the cells but not so long that it caused off-target activity. But, according to Lian, modRNA introduced a new problem.
When the modRNA Cas9 is successfully delivered to the target gene, it causes a double-stranded break in the genome, which some cells attempt to repair. Those who repair themselves can pass on the repair, or “mutation,” to their offspring. Researchers want to better understand this process, so these are the cells they want to harvest and study. The problem, according to Lian, is that most cells with this break recognize it as a major problem with the genome and will self-destruct rather than try to repair themselves.
To reduce the toxic side effects of Cas9 and help edited cells survive, Lian’s team introduced a small protein known to help cells grow. According to Lian, this added protein inhibited cell death and improved Cas9 editing efficiency up to 84%.
The researchers also found that the modRNA could improve other gene editing techniques, such as base editing. Base editing can knock out genes or correct mutations in the genome by using a protein to change a single nucleotide instead of cutting both strands, as CRISPR does.
“We transfected stem cells with either a plasmid-based base editing protein or a modRNA-based base editing protein,” Lian explained. “Our modRNA-based method was more than four times more efficient in successfully editing the genome, at 68%, than the plasmid-based technique, at about 16%.”
Researchers will be able to better understand genes and their functions as more gene-editing labs improve their efficiency and effectiveness, according to Lian.
“The human body has over 20,000 genes, but we only study about 10% of them,” Lian explained. “Exploring the function of each remaining gene one at a time could take a lifetime; however, using engineered stem cells derived from our highly efficient gene-editing techniques can greatly accelerate this process.”