Used lithium-ion batteries from cell phones, laptops, and an increasing number of electric vehicles are piling up, but the only ways to recycle them are to burn or chemically dissolve shredded batteries. Current state-of-the-art methods can pose environmental challenges and be difficult to commercialize on a large scale.
The traditional method recovers only a small percentage of the battery materials and relies on caustic, inorganic acids, and hazardous chemicals that may introduce impurities. It also necessitates complex separation and precipitation processes to recover the critical metals. However, recovering metals such as cobalt and lithium have the potential to reduce pollution as well as reliance on foreign sources and clogged supply chains.
Researchers at the Department of Energy’s Oak Ridge National Laboratory have improved on approaches that dissolve the battery in a liquid solution in order to reduce the amount of hazardous chemicals used in the process. This simple, efficient, and environmentally friendly solution developed by ORNL researchers overcomes the main obstacles presented by previous approaches.
Our approach could reduce the cost of batteries over time. The research was conducted in ORNL’s Battery Manufacturing Facility, the country’s largest open-access battery manufacturing research and development center.
Yaocai Bai
The spent battery is immersed in a solution of organic citric acid dissolved in ethylene glycol, an antifreeze agent commonly found in consumer products such as paint and makeup. Citric acid is derived from renewable resources and is far safer to handle than inorganic acids. This environmentally friendly solution resulted in a remarkably efficient separation and recovery process for metals from the battery’s positively charged electrode, known as the cathode.
“Because the cathode contains the critical materials, it is the most expensive part of any battery, contributing more than 30% of the cost,” explained Yaocai Bai, a member of the ORNL battery research team. “Our approach could reduce the cost of batteries over time.” The research was conducted in ORNL’s Battery Manufacturing Facility, the country’s largest open-access battery manufacturing research and development center.
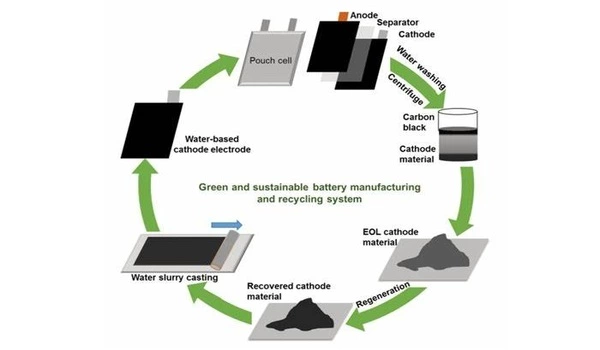
The recycling technique developed there leached nearly 100% of the cobalt and lithium from the cathode without introducing impurities in the system. It also enabled efficient separation of the metal solution from other residues. Most importantly, it served a second function by recovering over 96% of the cobalt in a matter of hours, without the typical addition of more chemicals in what is usually a tricky process of manually balancing acid levels.
“This is the first time that one solution system has covered the functions of both leaching and recovery,” said lead researcher Lu Yu. “It was thrilling to discover that the cobalt would precipitate and settle out on its own.” That was not what we expected.”
Eliminating the need for additional chemicals lowers costs and avoids the production of byproducts or secondary waste. “We are pleased that our scientists’ recycling process can pave the way for greater recovery of battery critical materials,” said Ilias Belharouak, corporate fellow and head of ORNL’s electrification section.
The leaching performance of citric acid and ethylene glycol has previously been investigated, but that method used more acid and a lower temperature, which proved less effective, according to Bai.
“We were surprised by how quickly the leaching happened in our solution,” Bai told me. “With an organic acid, it usually takes 10-12 hours, but this took only one.” Conventional inorganic acid solutions are also slower because they contain water, which has a boiling point that limits the temperature of the reaction.