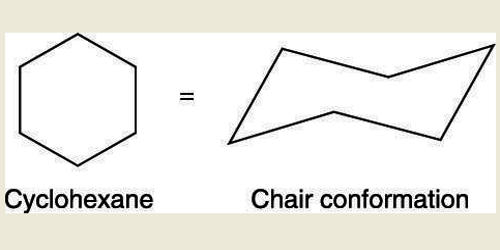
A cyclohexane conformation is any of several three-dimensional shapes adopted by a cyclohexane molecule. Because many compounds feature structurally similar six-membered rings, the structure and dynamics of cyclohexane are important prototypes of a wide range of compounds. It can refer to many different 3-Dimensional shapes assumed by a cyclohexane molecule without disturbing the integrity of the chemical bonds in it.
Cyclopropane is necessarily planar (flat), with the carbon atoms at the corners of an equilateral triangle. The internal angles of a flat regular hexagon are 120°, while the preferred angle between successive bonds in a carbon chain is about 109.5°, the tetrahedral angle. The 60º bond angles are much smaller than the optimum 109.5º angles of a normal tetrahedral carbon atom, and the resulting angle strain dramatically influences the chemical behavior of this cycloalkane. However, the carbon-carbon bonds belonging to the cyclohexane ring have a tetrahedral symmetry, with the bond angles corresponding to 109.5o. Therefore, the cyclohexane ring tends to assume certain non-planar (warped) conformations, which have all angles closer to 109.5° and therefore lower strain energy than the flat hexagonal shape. If two carbon atoms on opposite sides of the six-membered ring are lifted out of the plane of the ring, much of the angle strain can be eliminated.
The bond angles would necessarily be 120º, 10.5º larger than the ideal tetrahedral angle. Also, every carbon-carbon bond in such a structure would be eclipsed. The most important shapes are chair, half-chair, boat, and twist-boat. Their relative stabilities are: chair > twist boat > boat > half-chair. All relative conformational energies are shown below. The molecule can easily switch between these confirmations, and only two of them—chair and twist-boat—can be isolated in pure form. This geometry of chair cyclohexane conformations is generally preserved when the hydrogen atoms are replaced by halogen atoms such as fluorine, chlorine, bromine, and iodine. Examples of common conformations of cyclohexane include the boat, the twist-boat, the chair, and the half-chair conformations, which are named based on the shape that the cyclohexane molecule assumes in them. When chair flipping occurs, axial carbon-hydrogen bonds become equatorial and the equatorial carbon-hydrogen bonds become axial. However, they retain the corresponding ‘up’ or ‘down’ positions. The lowest energy conformation is chair conformation; thus, it is the most popular. The boat conformation of cyclohexane is not a very stable form due to the torsional strain applied to the cyclohexane molecule. These twist-boat conformations of cyclohexane are much more stable than their boat-shaped counterparts.