1,2,3-Cyclohexatriene, also known as benzyne or phenylenediyne, is an organic compound with the molecular formula C6H4. It consists of a planar ring of six carbon atoms with alternating single and triple bonds.
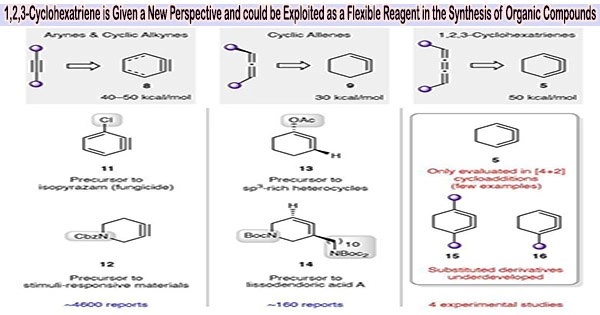
The 1,2,3-cyclohexatriene, an isomer of benzene, has been discovered to have the potential to be a versatile reagent in organic synthesis by a group of chemists at the University of California, Los Angeles. Andrew Kelleghan, Ana Bulger, Dominick Witkowski, and Neil Garg experimented with the high-energy substance for their study, which was published in the journal Nature.
Since it was thought to be too unstable to be effective, 1,2,3-cyclohexatriene has been essentially neglected as a suitable reagent for use in chemical synthesis for a long time. Nevertheless, it is promising because it has a lot more energy than benzene.
1,2,3-Cyclohexatriene can undergo various reactions due to the high energy associated with the strained triple bonds. It can undergo cycloaddition reactions, Diels-Alder reactions, and electrophilic addition reactions. The reactive nature of benzyne makes it a useful synthetic intermediate in organic chemistry.
Additionally, because of their potential to synthesize valuable organic compounds, isomers of benzene in general have long piqued the curiosity of organic chemists. In this new study, the researchers hypothesised that the isomer 1,2,3-cyclohexatriene would show to be advantageous as a reagent for synthesizing a variety of organic molecules under the proper circumstances.
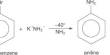
First, the researchers started making samples using a technique created in the 1990s to create intermediates out of a precursor. They then employed a variety of approaches to create a wide range of fused-ring adducts, all of which were probably to react well with nucleophiles, similar to benzenes. After that, they got to work testing the substance to see if it would make an effective reagent.
The team ran a variety of trapping reactions with dimethyl trienes to explore the viability of utilizing it as an electrophilic reagent. Then, they demonstrated nucleophilic reactions using sily1 group members and carried out reactions to demonstrate the possibility of σ-bond insertions.
Despite its high degree of reactivity and short lifetime, the group also carried out computational investigations in conjunction with experimental research that demonstrated the usage of 1,2,3-cyclohexatriene derivatives in specific processes.
Additionally, the scientists demonstrated that 1,2,3-cyclohexatrienes could be employed in lengthy synthetic sequences, demonstrating their usefulness in the construction of stereochemically and topologically complicated compounds. They come to the conclusion that these isomers and their derivatives merit additional research for usage in the production of organic products.