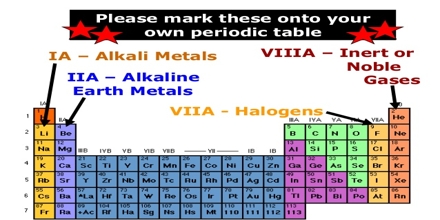
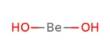
Basic objective of this lecture is to present Alkali Metals and the Alkaline Earth metals. The Group 1 elements have a specific family name—alkali metals. Each alkaline earth metal is denser and harder and has a higher melting point than the alkali metal in the same period. Alkaline earth metals are reactive, but not as reactive as the alkali metals. Here also briefly explain Groups 1 and 2 are always found in nature combined with other elements and why they’re called active metals because of their readiness to form new substances with other elements.
Alkali Metals and the Alkaline Earth metals