Xanthan gum (/ˈzaenθən/) is a long-chain polysaccharide produced by combining fermented sugars (glucose, mannose, and glucuronic acid) with bacteria. It works as a thickener, emulsifier, and stabilizer, preventing ingredients from separating.
Xanthan gum is a common food addition used as a thickening or stabilizer. Allene Rosalind Jeanes and her research team at the United States Department of Agriculture found it, and CP Kelco commercialized it under the brand name Kelzan in the early 1960s.
Emulsions, foams, and suspensions are thickened and stabilized using it. Xanthan gum gets its name from the bacterium Xanthomonas campestris, which is employed in the fermentation process. The bacterium that causes black rot on broccoli, cauliflower, and other green vegetables is the same one that causes it.
The extracellular polysaccharide xanthan gum is generated on a nutritive medium including glucose, NH4Cl, a variety of amino acids, and minerals by Xanthomonas campestris and some similar microbes. Isopropanol precipitation in the presence of KCl recovers the polysaccharide from the medium.
When xanthan gum powder is mixed with water, it disperses fast and forms a viscous, stable solution. For many products, this makes it an excellent thickening, suspending, and stabilizing ingredient. The USP28/NF and the PhEur both have a monograph on xanthan gum. It is soluble in hot and cold water and stable in acidic and alkaline (pH 5–13) environments.
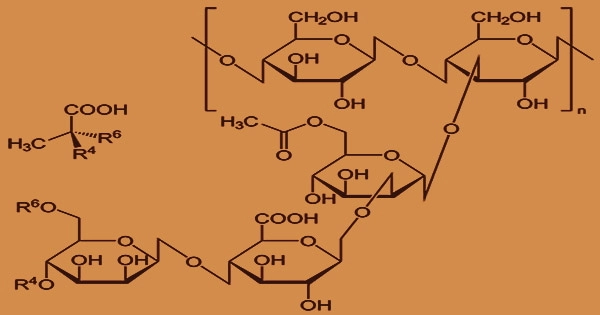
The fermentation of glucose and sucrose produces it. A sterile aqueous solution of carbohydrate(s), a source of nitrogen, dipotassium phosphate, and some trace elements make up the polysaccharide medium for X. campestris cultivation. D-glucose and D-mannose are the major hexose units in xanthan gum’s fundamental structure, together with D-glucuronic acid.
Two D-mannose residues and one D-glucuronic acid residue appear as mixed K+, Na+, and Ca++ salts in the trisaccharide side chain. The charged trisaccharide side chains are hypothesized to form a quaternary structure by the interaction of xanthan molecules. Xanthan gum is a polymer that dissolves in water.
There is no HPLC method for determining identification or purity in the USP or PhEur. The medium is aerated and agitated, and the xanthan polymer is formed extracellularly. It is made up of pentasaccharide repeat units with the molar ratios 2:2:1 of glucose, mannose, and glucuronic acid.
It’s a soluble fiber, despite being created in a lab. Soluble fibers are carbohydrates that our bodies are unable to break down. In our digestive tract, they absorb water and transform into a gel-like substance, slowing digestion. A strain of X. campestris that can thrive on lactose has been created, allowing it to be used to digest whey, a waste product from the dairy industry.
Xanthan gum is a food additive that is commonly used to modify the rheological qualities of a variety of foods. In toothpaste and pharmaceutical manufacture, xanthan gum is utilized as a thickening and stabilizing agent. It is used to manufacture diabetes medications that decrease blood sugar and total cholesterol.
Laxatives are made from xanthan gum. In patients with dry mouth, it’s sometimes used as a saliva substitute. On May 20, 2011, the FDA issued a press release alerting parents, caregivers, and health care providers not to feed SimplyThick to premature infants, a food thickening additive using xanthan gum as the active component.
Many personal care and beauty products contain xanthan gum. It enables these items to be thick while still flowing freely from their containers. It also enables the suspension of solid particles in liquids. A fermentation of carbohydrate and Xanthomonas campestris produces this gum.
Xanthan gum is utilized in concentrations of 0.5 percent or less in most foods. Sauces, dressings, meat and poultry items, bread and confectionery products, beverages, dairy products, and a variety of other foods contain it. Oxidizing agents, certain tablet film coatings, carboxymethylcellulose sodium, dried aluminum hydroxide gel, and some active substances like amitriptyline, tamoxifen, and verapamil are incompatible with xanthan gum.