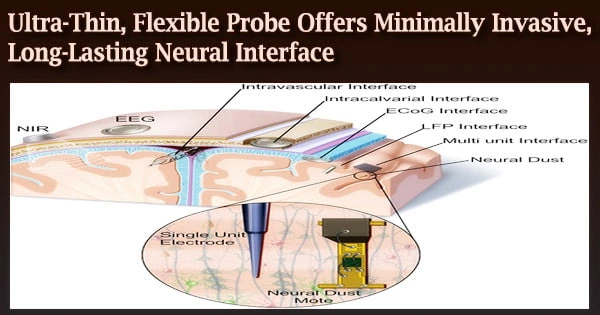
Researchers from the Salk Institute for Biological Studies and the University of California, San Diego have created a tiny neural probe that may be implanted for extended lengths of time to record and stimulate neural activity while causing the least amount of damage to the surrounding tissue.
The novel brain probe is described in a report that appeared in Nature Communications on June 7 and is both flexible and incredibly thin about one-fifth the breadth of a human hair. According to the research team, this kind of neural probe would be suitable for researching delicate and dynamic regions of the nervous system, such as the spinal cord or peripheral nerves.
“This is where you’d need a really small, flexible probe that can fit in between vertebrae to interface with neurons and can bend as the spinal cord moves,” said Axel Nimmerjahn, associate professor at the Salk Institute and co-senior author of the study.
These characteristics also increase its compatibility with biological tissue and decrease its propensity to elicit an immunological response, making it appropriate for long-term use.
“For chronic neural interfacing, you want a probe that’s stealthy, something that the body doesn’t even know is there but can still communicate with neurons,” said study co-senior author Donald Sirbuly, professor of nanoengineering at the UC San Diego Jacobs School of Engineering.
While there are other flexible, ultra-thin probes available, this tiny probe stands out because it can both monitor the electrical activity of neurons and use light to trigger particular groups of neurons.
“Having this dual-modality electrical recording and optical stimulation in such a small footprint is a unique combination,” said Sirbuly.
An electrical channel and an optical channel make up the probe. An extremely thin polymer electrode is present in the electrical channel. A thin optical fiber is also present in the optical channel. These two channels needed to be combined with some smart engineering.
This is where you’d need a really small, flexible probe that can fit in between vertebrae to interface with neurons and can bend as the spinal cord moves.
Axel Nimmerjahn
To keep the channels from interfering with one another, the researchers had to figure out how to isolate them and fit them both into a small probe with a diameter of only 8 to 14 micrometers. They also had to make sure that the device was mechanically flexible, durable, biocompatible, and capable of performing on par with cutting-edge neural probes.
In order to manufacture the probe, the appropriate materials had to be combined, and the fabrication of the electrical channel had to be optimized. For up to a month, the team placed the probes in the live mice’s brains.
After extended implantation, the probes barely generated any inflammation in the brain tissue. The probes were highly sensitive to detect electrical activity from neurons while the mice roamed around in a regulated environment.
In order to trigger particular physical reactions, the probes were also utilized to target particular neuron types. The scientists activated neurons in the cortex of the mice to cause them to wiggle their whiskers using the optical channels of the probes.
The purpose of these investigations on brain tissue was to demonstrate the theory. The group intends to use their probe in next spinal cord research.
“Currently, we know relatively little about how the spinal cord works, how it processes information, and how its neural activity might be disrupted or impaired in certain disease conditions,” said Nimmerjahn.
“It has been a technical challenge to record from this dynamic and tiny structure, and we think that our probes and future probe arrays have the unique potential to help us study the spinal cord not just understand it on a fundamental level, but also have the ability to modulate its activity.”
The neural probe technology discussed in this article has been the subject of a patent application from UC San Diego and the Salk Institute.
The Defense Advanced Research Projects Agency (DARPA) Biological Technologies Office Electrical Prescriptions program (HR0011-16-2-0027), the UC San Diego Kavli Institute for Brain and Mind (2018-1492), and the National Institutes of Health (R01 NS108034, U19 NS112959, U01 NS103522, and P30 CA014195), supported this work.
This work was done in part at the National Nanotechnology Coordinated Infrastructure, which is funded by the National Science Foundation (grant ECCS-1542148), and the San Diego Nanotechnology Infrastructure (SDNI) at UC San Diego.