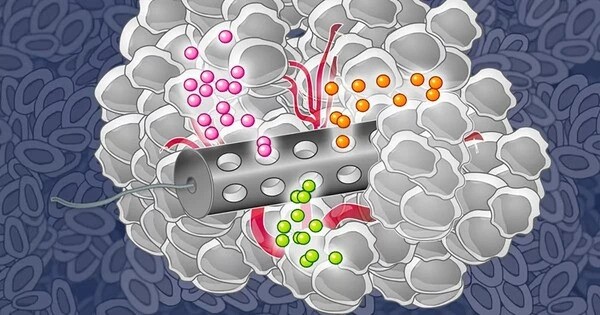
Multiple rounds of various treatments are frequently required for patients with late-stage cancer, which may not always be helpful and may result in unintended adverse effects. Researchers at MIT have created microscopic particles that may be implanted at a tumor site to provide two forms of therapy—heat and chemotherapy—in an effort to increase the treatment options for those patients.
The synergistic effect of the two medicines may prolong the patient’s lives longer than administering one medication at a time, and this strategy may avoid the side effects that frequently accompany intravenous chemotherapy. In a mouse trial, the researchers demonstrated that this treatment greatly increased the survival of the animals and totally removed tumors in the majority of the animals.
“One of the examples where this particular technology could be useful is trying to control the growth of really fast-growing tumors,” says Ana Jaklenec, a principal investigator at MIT’s Koch Institute for Integrative Cancer Research. “The goal would be to gain some control over these tumors for patients that don’t really have a lot of options, and this could either prolong their life or at least allow them to have a better quality of life during this period.”
Jaklenec is one of the senior authors of the new study, along with Angela Belcher, the James Mason Crafts Professor of Biological Engineering and Materials Science and Engineering and a member of the Koch Institute, and Robert Langer, an MIT Institute Professor and member of the Koch Institute. Maria Kanelli, a former MIT postdoc, is the lead author of the paper, which appears in the journal ACS Nano.
One of the examples where this particular technology could be useful is trying to control the growth of really fast-growing tumors. The goal would be to gain some control over these tumors for patients that don’t really have a lot of options, and this could either prolong their life or at least allow them to have a better quality of life during this period.
Ana Jaklenec
Dual therapy
Chemotherapy, surgery, and radiation therapy are frequently used in conjunction for patients with advanced malignancies. A more recent method of treatment is phototherapy, which entails injecting or implanting particles that have been heated by an external laser to a point where the tumor cells are killed nearby without causing damage to adjacent tissue.
Gold nanoparticles, which release heat when exposed to near-infrared light, are used in current phototherapy techniques in clinical trials.
In order to facilitate the patient’s experience during treatment and maybe have synergistic effects, the MIT team set out to develop a method of delivering chemotherapy and phototherapy together. They chose to employ molybdenum sulfide, an inorganic substance, as the phototherapeutic agent. This material converts laser light to heat very efficiently, which means that low-powered lasers can be used.
The researchers coupled molybdenum disulfide nanosheets with either doxorubicin, a hydrophilic medicine, or violacein, a hydrophobic agent, to construct a microparticle that could deliver both of these therapies. The chemotherapeutic and molybdenum disulfide are combined with a polymer known as polycaprolactone to create the particles, which are then dried into a film that can be formed into microparticles of various sizes and shapes.
The team made 200 micrometer-wide cubic particles for this investigation. The particles stay in the tumor site for the duration of the treatment after being administered there. The particles are heated by an external near-infrared laser during each treatment cycle. This laser has a limited impact on the tissue and can reach depths of a few millimeters to centimeters.
“The advantage of this platform is that it can act on demand in a pulsatile manner,” Kanelli says. “You administer it once through an intratumoral injection, and then using an external laser source you can activate the platform, release the drug, and at the same time achieve thermal ablation of the tumor cells.”
The researchers employed machine-learning algorithms to determine the optimal laser intensity, irradiation duration, and phototherapeutic agent concentration in order to optimize the therapy regimen. This prompted them to create a roughly three-minute laser treatment cycle. The particles are heated to roughly 50 degrees Celsius at that period, which is hot enough to destroy tumor cells. A portion of the chemotherapy medication contained in the particles is released when the polymer matrix inside them melts at this temperature as well.
“This machine-learning-optimized laser system really allows us to deploy low-dose, localized chemotherapy by leveraging the deep tissue penetration of near-infrared light for pulsatile, on-demand photothermal therapy. This synergistic effect results in low systemic toxicity compared to conventional chemotherapy regimens,” says Neelkanth Bardhan, a Break Through Cancer research scientist in the Belcher Lab, and second author of the paper.
Eliminating tumors
The researchers tested the microparticle treatment in mice that were injected with an aggressive type of cancer cells from triple-negative breast tumors. Once tumors formed, the researchers implanted about 25 microparticles per tumor, and then performed the laser treatment three times, with three days in between each treatment.
“This is a powerful demonstration of the usefulness of near-infrared-responsive material systems,” says Belcher, who, along with Bardhan, has previously worked on near-infrared imaging systems for diagnostic and treatment applications in ovarian cancer. “Controlling the drug release at timed intervals with light, after just one dose of particle injection, is a game changer for less painful treatment options and can lead to better patient compliance.”
The tumors were totally removed in the mice that got this treatment, and they lived a lot longer than the mice that got chemotherapy, phototherapy, or no treatment at all. Additionally, mice that had all three treatment cycles fared significantly better than mice that just received one laser therapy.
The particles’ biocompatible polymer has already received FDA approval for use in medical devices. In order to eventually assess the particles in clinical trials, the researchers now intend to examine them in larger animal models. They anticipate that any solid tumor, even those that have spread, may benefit from this treatment.