The origins of life are shrouded in mystery. While the complicated dynamics of mitosis are well understood in somatic cells (specialized cells such as skin and muscle cells), they remain enigmatic in our bodies’ initial cells, embryonic cells. Embryonic mitosis is notoriously difficult to investigate in vertebrates, as live functional studies and imaging of experimental embryos are technically limited, making it difficult to track cells throughout development.
However, researchers from the Cell Division Dynamics Unit at the Okinawa Institute of Science and Technology (OIST) have recently published a paper in Nature Communications, together with Professors Toshiya Nishimura from Hokkaido University (previously at Nagoya University), Minoru Tanaka from Nagoya University, Satoshi Ansai from Tohoku University (currently at Kyoto University), and Masato T. Kanemaki from the National Institute of Genetics. The study takes the first major steps towards answering questions about embryonic mitosis, thanks to a combination of novel imaging techniques, CRISPR/Cas9 genome editing technology, a modern protein-knockdown system, and medaka, or Japanese rice fish (Oryzias latipes).
The timelapses they’ve created assist answer fundamental concerns regarding the complex process of evenly splitting chromosomes during embryonic mitosis while also charting the next frontier of scientific discovery. According to the study’s senior author, Professor Tomomi Kiyomitsu, “they are beautiful, both on their own and because they lay a new foundation for elucidating embryonic mitosis.”
With this paper, we have created a solid foundation, but we have also opened a new frontier. Embryonic mitosis is beautiful, mysterious, and challenging to study, and we hope that with our work, we can eventually get a little closer to understanding the intricate processes at the beginning of life.
Professor Kiyomitsu
Watch Professor Kiyomitsu explain the beautiful timelapses here: https://youtu.be/HeEp1pmgWgk
The critical moment in embryonic mitosis, when the chromosomes, which contain all of the cell’s genetic information, are aligned and separated equally into daughter cells, is central to the riddle. The mitotic spindle, which is made up of microtubules (long protein fibers utilized for intracellular structure and transport), radiates from the spindle’s opposite poles and binds to the chromosomes in the middle, is an important player in this process.
During division, the spindle catches duplicated chromosomes and evenly distributes them throughout the daughter cells. There are numerous factors that influence spindle formation, one of which is the protein Ran-GTP, which is essential for cell division in female reproductive cells, which lack centrosomes (cell organelles responsible for assembling microtubules), but not in small somatic cells, which do have centrosomes. However, it has long been uncertain if Ran-GTP is essential for spindle assembly in vertebrate early embryos, which have centrosomes but exhibit distinct characteristics such as increased cell size.
In contrast to mammalian early embryos, embryonic cells in fish are transparent and develop synchronously in a uniform, single-cell layer sheet, which makes them significantly easier to track. The medaka turned out to be particularly well-suited for the researchers, as these fish tolerate a wide range of temperatures, produce eggs daily, and have a relatively small genome. Being temperature-tolerant means that the medaka embryonic cells could survive at room temperature, making them particularly suited for long, live timelapse photography.
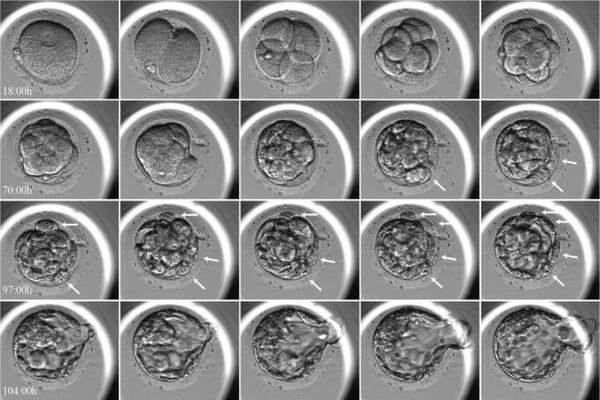
Medaka are ideal candidates for CRISPR/Cas9-mediated genome editing due to their prolific egg production and relatively small genome size. Using this approach, the researchers generated genetically modified, or transgenic, medaka whose embryonic cells physically highlight the movements of specific proteins involved in mitosis.
The researchers observed that huge early embryos form unique spindles that are distinct from somatic spindles while investigating timelapses of the growing mitotic spindle in live, transgenic medaka embryos. Furthermore, Ran-GTP is essential for spindle formation in early embryonic divisions, although its relevance decreases in later stages. This could be because the spindle structure is changed when cells shrink throughout development, but the specific explanation is still unknown.
The researchers also discovered that the early embryonic cells do not have a dedicated spindle assembly checkpoint, which characterizes most somatic cells, and which serves to ensure that the chromosomes are properly aligned before segregation. As Professor Kiyomitsu surmises, “the checkpoint is not active, and yet the chromosome segregations are still very accurate. This could be explained by the fact that embryonic cells need to divide very quickly, but it is something that we want to study further.”
While genetically manipulating the medaka fish and examining the early embryos has provided fresh insights into embryonic mitosis, Professor Kiyomitsu and his team are only getting started. In addition to queries about the decreasing role of Ran-GTP in later stages and the absent spindle assembly checkpoint, he highlights the pleasing symmetry of cell divisions in the timelapses: “The spindle development is characterized by a high degree of symmetry, since the cells appear to divide in specific sizes and directions, and the spindle is always in the middle of the cells. How does the spindle arrange itself so consistently across the cells, and how does it find the center every time?”
Moving beyond the timelapses, the team intends to strengthen this new foundation by adding more medaka gene lines to serve as models for embryonic cell research while also optimizing the genome editing procedure. Eventually, the team hopes to test the generalizability of their findings by studying embryonic mitosis in other organisms, and at a later stage, they hope to investigate the evolution of spindle assembly and embryonic divisions, which would also contribute to a better understanding of human embryogenesis and the development of diagnostic and treatment methods for human infertility.
“With this paper, we have created a solid foundation,” summarizes Professor Kiyomitsu, “but we have also opened a new frontier. Embryonic mitosis is beautiful, mysterious, and challenging to study, and we hope that with our work, we can eventually get a little closer to understanding the intricate processes at the beginning of life.”