Thermal composition, or thermolysis, is a chemical decomposition caused by heat. It is the breakdown of molecules by the action of heat. It is a process of burning out the organic components such as binders and dispersants. The decomposition temperature of a substance is the temperature at which the substance chemically decomposes. Incomplete binder removal and uncontrolled thermolysis may introduce membrane defects. The reaction is usually endothermic as heat is required to break chemical bonds in the compound undergoing decomposition. The term pyrolysis is often used in place of thermolysis when organic compounds are decomposed at high temperatures. If decomposition is sufficiently exothermic, a positive feedback loop is created producing thermal runaway and possibly an explosion or other chemical reactions. In a combination reaction, a substance is formed as a result of chemical combination, while in a decomposition reaction, the substance breaks into new substances. If the reaction is exothermic, the reaction proceeds spontaneously after initiation, which sometimes causes an explosion.
Examples
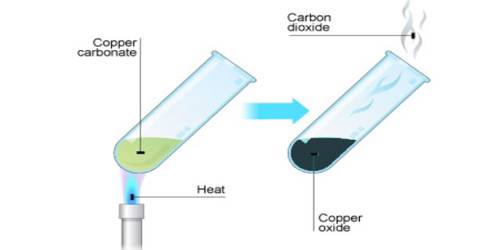
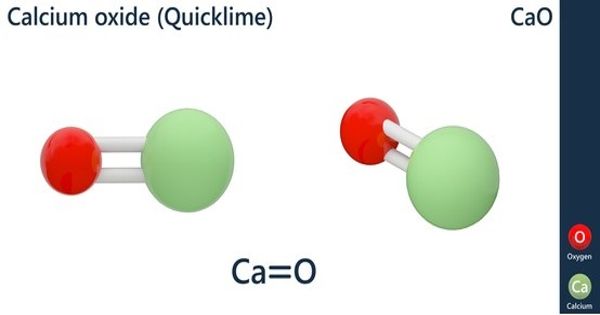
- Calcium carbonate (limestone or chalk) decomposes into calcium oxide and carbon dioxide when heated. The chemical reaction is as follows: CaCO3 → CaO + CO2 (All metal carbonates decompose to give the metal oxide and carbon dioxide).
- The reaction is used to make quick lime, which is an industrially important product. Other example of thermal Decomposition is :- 2Pb(NO3)2 —-> 2PbO + O2 +4NO2
- Some oxides, especially of weakly electropositive metals decompose when heated to high enough temperature. A classical example is the decomposition of mercuric oxide to give oxygen and mercury metal. The reaction was used by Joseph Priestley to prepare samples of gaseous oxygen for the first time.
- When water is heated to well over 2000 °C, a small percentage of it will decompose into OH, monatomic oxygen, monatomic hydrogen, O2, and H2.
- The compound with the highest known decomposition temperature is carbon monoxide at ≈3870 °C (≈7000 °F).