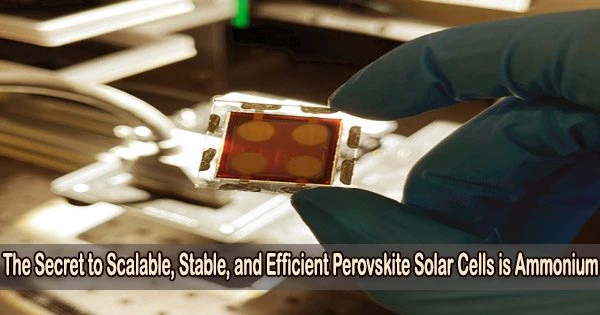
The first successful application of lead acetate as a precursor in the fabrication of formamidinium-caesium perovskite solar cells has shown a new route to the development of robust, effective perovskite photovoltaics at industrial scale.
The best outcomes ever seen for a device constructed from a non-halide lead source were achieved by members of Exciton Science, based at Monash University, who were able to produce perovskite solar cells with a 21% efficiency.
A mini prototype solar panel featuring these cells achieved 18.8% efficiency. The large-area perovskite layer was created in ambient air using a single-step blade coating, proving the technology’s potential for use in large-scale manufacturing.
The test devices also showed strong thermal stability, continuing to function with no efficiency loss after 3,300 hours running at 65°C.
First author Jie Zhao, a PhD student at Monash University, said: “We’ve been able to use lead acetate in a one-step, spin-coating process to get the perfect, high-quality formamidinium-caesium perovskite thin film.”
“And because we don’t need an anti-solvent agent, we can do this via large-scale techniques, such as blade coating, which means it’s viable at industrial scale.”
The results have been published in the journal Energy & Environmental Science.
Corresponding author and Monash University colleague Dr. Wenxin Mao said: “The vast majority of perovskite solar cell research uses lead halides, particularly lead iodide. The lead iodide needs to be 99.99% pure and it’s very expensive to synthesise cells using lead iodide.”
“We’re the first group to make highly stable formamidinium-cesium perovskite solar cells using lead acetate rather than lead iodide.”
“We have provided the entire research community a second way to make high-quality perovskite solar cells.”
We’ve been able to use lead acetate in a one-step, spin-coating process to get the perfect, high-quality formamidinium-caesium perovskite thin film. And because we don’t need an anti-solvent agent, we can do this via large-scale techniques, such as blade coating, which means it’s viable at industrial scale.
Jie Zhao
More about perovskites: Promise versus problems
Due to their flexible design, low production cost, and adjustable band gap in comparison to silicon, thin film solar cells based of perovskites have the potential to upend the solar energy industry.
Researchers are having trouble addressing dependability problems, and they also need to figure out how to produce devices at a scale that would be profitable for businesses.
Perovskites are solution processed (made in liquid) using a variety of different ingredients.
The majority of methods use lead halides and call for the use of potent polar solvents with high boiling points as well as antisolvent quenching agents to regulate the crystallization of perovskite.
Due to faults in the thin films caused by this intricate system, the final device loses efficiency very quickly. It’s also hard to control.
Due to its ability to produce ultrasmooth thin films with fewer flaws, the chemical compound lead acetate has emerged as a possible alternative precursor.
Only methylammonium or cesium-based perovskites, which are relatively unstable and unsuitable for practical uses, have previously been produced using lead acetate.
Due to their improved stability, perovskites produced from formamidinium and caesium make a better option for industrial usage. Previous attempts to synthesise them using lead acetate as the precursor failed.
To investigate and solve this issue, the researchers, together with their collaborators at Wuhan University of Technology in China, examined the underlying molecular mechanisms.
Through X-ray diffraction and nuclear magnetic resonance spectroscopy, they identified the need to use ammonium as a volatile cation (positively charged ion) at a critical stage.
Contributing author Dr. Sebastian Fürer said: “The presence of ammonium served to drive away the residual acetate during annealing, without forming unwanted side products.”
The researchers are hoping that their work on the underlying chemistry driving precursor behavior would motivate more attention to be paid to scalable techniques of metal halide perovskite device synthesis and construction.