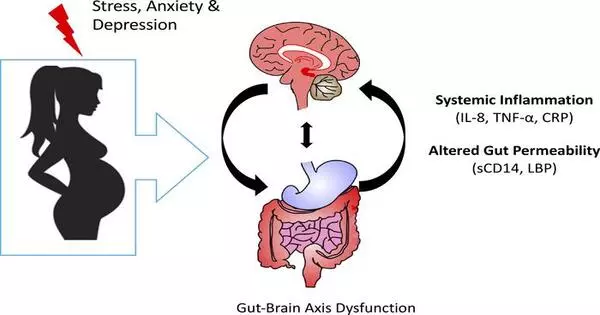
Maternal stress has been linked to an increased risk of premature birth. Premature babies frequently face health issues and may experience developmental delays. Maternal stress can contribute to infants having a lower birth weight. Low birth weight is linked to an increased risk of certain health issues, such as respiratory issues, weakened immune function, and developmental delays.
A new study from the University of Cincinnati investigates the impact of maternal stress during pregnancy on the neurodevelopment of babies. The findings were reported in the journal Molecular Psychiatry.
Prenatal maternal stress life events are linked to poor neurodevelopmental outcomes in offspring. The biological mechanisms underlying these associations are largely unknown, but a chemical reaction in the body called DNA methylation, in which a small molecule known as a methyl group is added to DNA, is thought to play a role. These findings may shed light on how the fetal environment influences not only neurodevelopment but also metabolism and immune function.
According to Anna Ruehlmann, a postdoctoral fellow in the Department of Environmental and Public Health Sciences at the UC College of Medicine and the study’s lead author, more than 5,500 people participated in the study, which was divided into 12 separate cohorts.
We discovered that when mom experienced a cumulative amount of stress during pregnancy, there was an association with DNA methylation in umbilical cord blood, which is a type of epigenetic modification in the developing baby in the womb.
Anna Ruehlmann
“Our study is the first to look at such a large sample size and examine the entire epigenome, so it’s not just looking at the stress control genes as in previous studies, it’s looking at all the epigenomic sites available right now that you can study,” she says.
The study looks at five different types of stress that expectant mothers face during their pregnancy. They are financial stress, conflict with a partner, conflict with a family member or friend, abuse (physical, emotional, and mental), and the death of a friend or relative, as well as a total score that combines all of the categories.
“We discovered that when mom experienced a cumulative amount of stress during pregnancy, there was an association with DNA methylation in umbilical cord blood, which is a type of epigenetic modification in the developing baby in the womb,” Ruehlmann says.
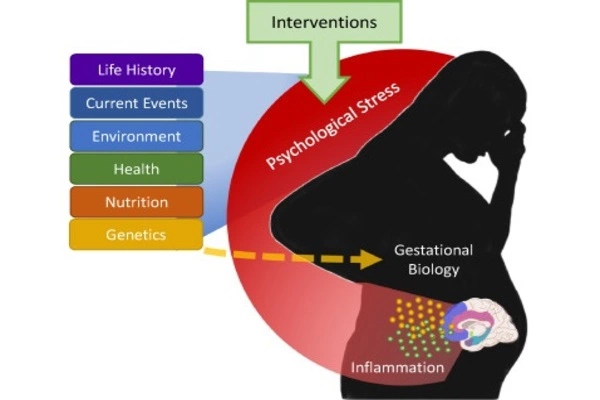
“An epigenetic modification does not change the sequence of the DNA, but the DNA is modified, which is dynamic and can change in response to environmental exposures.” As a result, it’s something that can be turned on or off later in the child’s life, or it may not do anything at all; it’s still unknown. It is thought to be a gene expression control mechanism.”
Another unknown, according to Ruehlmann, is how this process will affect children once they are born.
“We found five specific locations of DNA methylation with three different maternal stressors during pregnancy,” she explains. “One was cumulative stress and the stressor-specific domains of conflict with family/friends, abuse (physical, sexual, and emotional), and death of a close friend/relative, which were linked to DNA methylation in the developing fetus.” These were found in genes that have been linked to neurodevelopment. The next step is to conduct functional analyses to determine how these genes actually function and how DNA methylation affects their expression.”
Ruehlmann describes the procedure as a massive puzzle.
“Epigenetic modifications are a very dynamic process, and there are a lot of changes that can happen in response to environmental factors,” she says. “What you see biologically at the beginning of fetal development may not be seen as an outcome until later in a child’s development.” As a biologist, it’s fascinating to start uncovering some of the biological clues to how neurodevelopment is affected during fetal development. There are many puzzle pieces that have yet to be connected. It’s a lot of fun.”