A silent synapse is an excitatory glutamatergic synapse with NMDA-type glutamate receptors but no AMPA-type glutamate receptors in its postsynaptic membrane. These synapses are called “silent” because they lack normal AMPA receptor-mediated signaling, rendering the synapse inactive under normal conditions. Silent synapses are thought to be immature glutamatergic synapses.
Neuroscientists discovered that the adult brain contains millions of ‘silent synapses,’ or immature connections between neurons that remain dormant until they are recruited to aid in the formation of new memories. The adult brain contains millions of “silent synapses,” which are immature connections between neurons that remain inactive until they are recruited to help form new memories, according to MIT neuroscientists.
Until now, it was thought that silent synapses were only present during early development when they helped the brain learn new information. However, according to a new MIT study, approximately 30% of all synapses in the cortex of adult mice are silent.
According to the researchers, the existence of these silent synapses may help to explain how the adult brain is able to form new memories and learn new things without having to modify existing conventional synapses.
“These silent synapses are looking for new connections, and when important new information is presented, connections between the relevant neurons are strengthened. This lets the brain create new memories without overwriting the important memories stored in mature synapses, which are harder to change,” says Dimitra Vardalaki, an MIT graduate student and the lead author of the new study.
Mark Harnett, an associate professor of brain and cognitive sciences, is the senior author of the paper, which appears today in Nature. Kwanghun Chung, an associate professor of chemical engineering at MIT, is also an author.
These silent synapses are looking for new connections, and when important new information is presented, connections between the relevant neurons are strengthened. This lets the brain create new memories without overwriting the important memories stored in mature synapses, which are harder to change.
Dimitra Vardalak
A surprising discovery
Silent synapses were first observed in the brains of young mice and other animals by scientists decades ago. These synapses are thought to help the brain acquire the massive amounts of information that babies require to learn about their environment and how to interact with it during early development. These synapses were thought to disappear in mice after about 12 days of age (equivalent to the first months of human life).
However, some neuroscientists believe that silent synapses can survive into adulthood and help with memory formation. Animal models of addiction, which is thought to be primarily a disorder of aberrant learning, have provided evidence for this.
Theoretical work in the field from Stefano Fusi and Larry Abbott of Columbia University has also proposed that neurons must display a wide range of different plasticity mechanisms to explain how brains can both efficiently learn new things and retain them in long-term memory. In this scenario, some synapses must be established or modified easily, to form new memories, while others must remain much more stable, to preserve long-term memories.
In the new study, the MIT team did not set out specifically to look for silent synapses. Instead, they were following up on an intriguing finding from a previous study in Harnett’s lab. In that paper, the researchers showed that within a single neuron, dendrites – antenna-like extensions that protrude from neurons – can process synaptic input in different ways, depending on their location.
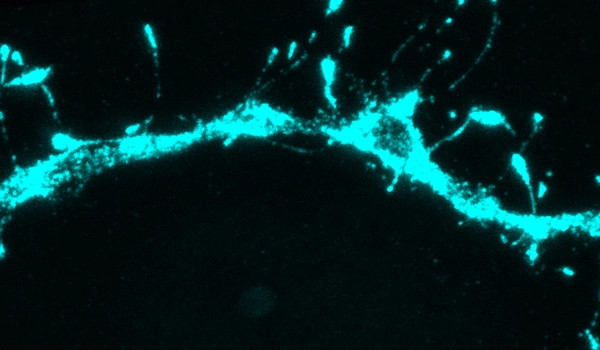
The researchers attempted to measure neurotransmitter receptors in different dendritic branches as part of that study to see if that would help account for the differences in their behavior. Chung’s eMAP (epitope-preserving Magnified Analysis of the Proteome) technique was used to accomplish this. Researchers can use this technique to physically expand a tissue sample and then label specific proteins in the sample, allowing them to obtain super-high-resolution images.
While they were imaging, they made an unexpected discovery. “The first thing we noticed, which was very strange and unexpected, was that there were filopodia everywhere,” Harnett says.
Filopodia, thin membrane protrusions that extend from dendrites, have previously been observed, but neuroscientists were unsure what they did. This is due in part to the fact that filopodia are so small that they are difficult to detect using traditional imaging techniques.
Following this discovery, the MIT team used the eMAP technique to search for filopodia in other parts of the adult brain. They were astounded to discover filopodia in the mouse visual cortex and other parts of the brain at a level ten times higher than previously observed. They also discovered that filopodia contained neurotransmitter receptors known as NMDA receptors but no AMPA receptors.
A typical active synapse has both of these types of receptors, which bind the neurotransmitter glutamate. NMDA receptors normally require cooperation with AMPA receptors to pass signals because NMDA receptors are blocked by magnesium ions at the normal resting potential of neurons. Thus, when AMPA receptors are not present, synapses that have only NMDA receptors cannot pass along an electric current and are referred to as “silent.”
Unsilencing synapses
To investigate whether these filopodia might be silent synapses, the researchers used a modified version of an experimental technique known as patch clamping. This allowed them to monitor the electrical activity generated at individual filopodia as they tried to stimulate them by mimicking the release of the neurotransmitter glutamate from a neighboring neuron.
Using this technique, the researchers found that glutamate would not generate any electrical signal in the filopodium receiving the input, unless the NMDA receptors were experimentally unblocked. This offers strong support for the theory that filopodia represent silent synapses within the brain, the researchers say.
The researchers also demonstrated that by combining glutamate release with an electrical current coming from the neuron’s body, they could “unsilence” these synapses. This combined stimulation causes the silent synapse to accumulate AMPA receptors, allowing it to form a strong connection with the nearby axon that is releasing glutamate.
The researchers discovered that converting silent synapses to active synapses was far easier than modifying mature synapses.
“That plasticity protocol doesn’t work if you start with an already functional synapse,” Harnett says. “Adult brain synapses have a much higher threshold, presumably because you want those memories to be fairly resilient. You don’t want them being overwritten all the time. Filopodia, on the other hand, can be recorded in order to create new memories.”
“Flexible and robust”
The findings offer support for the theory proposed by Abbott and Fusi that the adult brain includes highly plastic synapses that can be recruited to form new memories, the researchers say.
“This paper is, as far as I know, the first real evidence that this is how it actually works in a mammalian brain,” Harnett says. “Filopodia allow a memory system to be both flexible and robust. You need the flexibility to acquire new information, but you also need stability to retain the important information.”
The researchers are now looking for evidence of these silent synapses in human brain tissue. They also hope to investigate whether aging or neurodegenerative disease affects the number or function of these synapses.
“It’s entirely possible that by changing the amount of flexibility you have in a memory system, it could become much more difficult to change your behaviors and habits or incorporate new information,” Harnett says. “You could also imagine locating some of the molecular players involved in filopodia and attempting to manipulate some of those things in order to restore flexible memory as we age.”