A new study focuses on sulfur, a chemical element that, while known, has proven remarkably resistant to scientific efforts to investigate its role in the genesis of life.
Many artists have attempted to illustrate how the Earth would have looked billions of years ago, before life appeared. Many scenarios replace snow-covered mountains with lava-gushing volcanoes, and azure skies with lightning bolts striking what lies beneath from a hazy sky. But how did early Earth look? This question has been the focus of extensive scientific inquiry for decades.
A research led by Sukrit Ranjan, an assistant professor at the University of Arizona’s Lunar and Planetary Laboratory, focuses on sulfur, a chemical element that, while well-known, has proven remarkably resistant to scientific efforts to investigate its role in the beginning of life.
“Our picture of early Earth is pretty fuzzy,” said Ranjan, who studies sulfur concentrations in the planet’s oceans and atmosphere. He claims that the same processes that make our planet habitable, such as liquid water and plate tectonics, are continually destroying the rocks that preserve Earth’s geologic record. “It’s great for us because it recycles nutrients that would otherwise be locked up in Earth’s crust, but it’s terrible for geologists in the sense that it removes the messengers.”
Our picture of early Earth is pretty fuzzy. It’s great for us because it recycles nutrients that would otherwise be locked up in Earth’s crust, but it’s terrible for geologists in the sense that it removes the messengers.
Sukrit Ranjan
Published in the journal AGU Advances in December, Ranjan’s paper was selected as an editor’s highlight, in recognition of “experiments that were extremely difficult to perform but provide constraints for ongoing laboratory prebiotic chemistry experiments.” At the core of efforts to pull back the curtain on the emergence of life on Earth has been a concept known as the “RNA world,” Ranjan said, referring to ribonucleic acid, a class of molecules that are present in every living cell and crucial to life as we know it.
The RNA world hypothesis is based on an interesting feature of modern biology: of the four major categories of biomolecules — amino acids, carbohydrates, lipids, and nucleic acids — RNA is the only one that can act as an enzyme as well as store and replicate genetic information by making copies of itself. There’s just one problem: it’s really difficult to make.
“For about 50 years, people have tried to figure out how to make RNA without enzymes, which is how biology does it,” said Ranjan, adding that it wasn’t until the last five years that researchers discovered non-enzymatic RNA production routes.
“If we can get RNA, then on the far horizon we see a pathway to get everything else going,” he said. “And this begs the question: Was this molecule actually available earlier in any quantities whatsoever? And this is actually a major open question.”
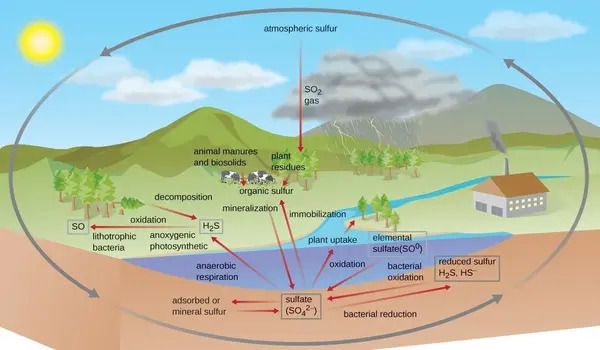
Scientists recently fulfilled a half-century quest to create RNA molecules without the use of biological enzymes, marking a significant step toward revealing the RNA world. However, each of these chemical routes relies on a crucial sulfur molecule known as sulfite. Scientists have discovered that there was lots of sulfur on the early, prebiotic Earth by investigating rock samples from some of the planet’s oldest formations. But how much of it remained in the atmosphere? How much of it ended up in the water? How much of it ended up as RNA-producing sulfite? These are the questions Ranjan and his team aimed to address.
“Once it’s in the water, what happens to it? Does it stick around for a long time, or does it go away quickly?” he said. “For modern Earth we know the answer — sulfite loves to oxidize, or react with oxygen, so it’ll go away super-fast.”
In contrast, geological evidence suggests that the early Earth’s atmosphere contained relatively little oxygen, allowing sulfite to collect and stay considerably longer. However, even in the absence of oxygen, sulfite is extremely reactive, and several reactions might have removed it from the early Earth environment.
One such reaction is disproportionation, which occurs when many sulfites react with one another, producing sulfate and elemental sulfur, both of which are unsuitable for origin-of-life chemistry. But how fast is this process? Would it have allowed for enough sulfites to accumulate to launch life?
“No one has actually looked into this in depth outside of other contexts, mainly wastewater management,” Ranjan said.
His team then set out to investigate this problem under various conditions, an effort that took five years from designing the experiments to publishing the results.
“Of all the atoms that stock the prebiotic shipyard, including carbon, hydrogen, nitrogen, oxygen, phosphorus and sulfur, sulfur is perhaps the thorniest,” wrote Sonny Harman of NASA’s Ames Research Center, in a viewpoint article accompanying the publication. Because of its eagerness to enter into chemical reactions, “sulfur compounds tend to be more unstable, posing hazards to lab personnel and equipment, clogging up instrumentation and gumming up experiments.”
A lab tech’s nightmare
In their setup, Ranjan and his co-authors dissolved sulfite in water at various levels of acidity or alkalinity, locked it into a container under an oxygen-free atmosphere and let it “age,” as Ranjan put it. Every week, the team measured the concentrations of various sulfites with ultraviolet light. At the end of the experiment, they subjected them to a suite of analyses, all geared toward answering a relatively simple question, he said: “Just how much of this original molecule is left, and what did it turn into?”
Sulfites, it found out, were much slower than popular wisdom suggested. Earlier research, for example, proposed a sulfur cloud encompassing the early Earth, but Ranjan’s team discovered that sulfites degrade more quickly under ultraviolet light than expected. In the absence of an ozone layer during Earth’s early days, this mechanism, known as photolysis, would have quickly removed sulfur compounds from the atmosphere and water, albeit not as efficiently as the abundant oxygen in today’s planet.
While it’s plausible that slow disproportionation could have allowed sulfites to accumulate, photolysis would have made that very unlikely except in certain environments such as shallow water pools, shaded from UV radiation, particularly if fed by surface runoff to provide mineral shields. Examples include underground pools or closed basin carbonate lakes, drainage-less depressions where sediments accumulate but water can only leave by evaporation.
“Think bodies of water like the Great Salt Lake in Utah or Mono Lake in California,” Ranjan said, adding that hydrothermal environments are emerging as hot candidates for life’s first appearance. Here, groundwater carrying dissolved minerals comes into contact with heat from volcanic activity, creating unique micro-environments that offer “safe spaces” for chemical process that could not occur elsewhere.
According to Ranjan, such areas can be found on land as well as at mid-ocean ridges in the deep water. “A modern-day example of this is Yellowstone National Park, where we find pools that accumulate lots of sulfite, despite the oxygen,” he went on to say, “and that can happen just because the sulfite is continually being replenished by volcanic outgassing.”
According to the scientists, the finding allows for the experimental testing of the notion of sulfite availability in the evolution of the first molecules of life. Ranjan is particularly enthused about one area of research: phylogenetic microbiology, which employs genomic analysis to recreate the blueprints of sulfur-using microbes thought to be among the oldest on Earth.
There is evidence that these bacteria obtain energy by converting highly oxidized forms of sulfur into less oxidized ones. Intriguingly, Ranjan noted that they rely on a rather complicated enzyme machinery for the first step, reducing sulfate, sulfur’s plentiful “modern” form, to sulfite, implying that these enzymes are the result of a lengthy evolutionary process. In contrast, only one enzyme is involved in the conversion of sulfite — the postulated essential component in “prebiotic puddle environments”—to sulfide.