A new generation of bioelectronic devices and sensors are now possible because to the development of a special microscopic toolbox of “green” adjustable electrical components.
Researchers from the University of Bristol have demonstrated how to create conductive, biodegradable wires from customized proteins in a work that was published in the Proceedings of the National Academy of Sciences (PNAS). These might work with the biological systems that all living things use to produce energy. These biological systems are typically made of iron or copper.
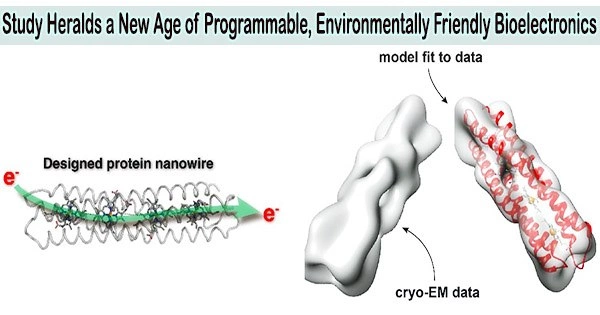
The tiny wires are a thousandth of the width of the finest human hair or the size of transistors on silicon chips. They are entirely composed of heme molecules, which are present in proteins like hemoglobin, which carries oxygen in red blood cells, and natural amino acids.
They were produced using harmless microorganisms, which eliminated the need for potentially difficult and environmentally harmful processes often employed in the manufacturing of synthetic compounds.
Lead author Ross Anderson, Professor of Biological Chemistry at the University of Bristol, said, “While our designs take inspiration from the protein-based electronic circuits necessary for all life on Earth, they are free from much of the complexity and instability that can prevent the exploitation of their natural equivalents on our own terms.”
“We can also build these minute electronic components to order, specifying their properties in a way that is not possible with natural proteins.”
This innovative new technique for creating custom proteins with adjustable electrical characteristics was developed in collaboration with top authorities in biomolecular engineering and simulation.
While our designs take inspiration from the protein-based electronic circuits necessary for all life on Earth, they are free from much of the complexity and instability that can prevent the exploitation of their natural equivalents on our own terms. We can also build these minute electronic components to order, specifying their properties in a way that is not possible with natural proteins.
Professor Ross Anderson
The multidisciplinary team created straightforward building pieces that could be assembled into larger, protein strands resembling wires for carrying electrons using cutting-edge computational methods.
They were able to visualize the structures of these wires using protein X-ray crystallography and electron cryo-microscopy (cryo-EM), techniques which allow structures to be viewed in the finest detail. Images of the tiniest individual protein ever analyzed were acquired using this method, pushing the technical limits of cryo-EM.
In the end, these nanoscale designer wires have the potential to be utilized in a variety of applications, such as biosensors for the detection of toxins in the environment and diseases.
Additionally, it is believed that this invention will serve as the basis for novel electrical circuits that can be used to make customized catalysts for green industrial biotechnology and synthetic photosynthetic proteins that can be used to harvest solar energy.
The breakthrough was part of a five-year project, entitled “The Circuits of Life,” involving the Universities of Bristol, Portsmouth, East Anglia, and University College London (UCL).
The team used its skills in protein design, electron transfer, biomolecular simulation, structural biology, and spectroscopy to acquire understanding of how electrons move through natural biological molecules, an essential mechanism that supports both photosynthesis and cellular respiration.
The initiative, which started last year, is projected to make greater advancements as it moves forward, offering important chances to assist with the switch to net zero and more sustainable industrial operations.
Co-author Adrian Mulholland, Professor of Chemistry at the University of Bristol, said, “These proteins show how protein design is increasingly delivering practically useful tools. They offer exciting possibilities as components for engineering biology and also are great systems for investigating the fundamental mechanisms of biological electron transfer.”