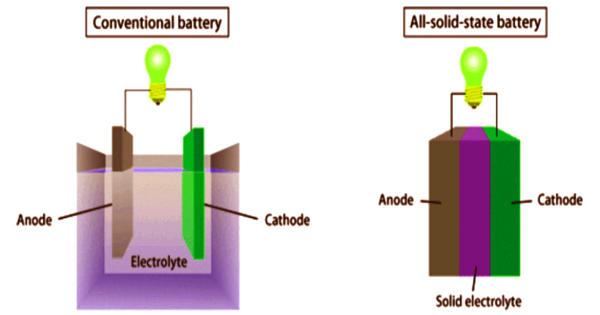
Solid-state batteries use both solid electrodes and electrolytes. They serve to be a potential alternative to conventional lithium-ion batteries, which use liquid or polymer electrolytes. A solid-state battery is a battery technology that uses solid electrodes and a solid electrolyte, instead of the liquid or polymer gel electrolytes found in lithium-ion or lithium polymer batteries. Solid-state batteries with two kinds of lithium solid electrolytes showed good characteristics for the graphite electrode. Materials proposed for use as solid electrolytes in solid-state batteries include ceramics (e.g. oxides, sulfides, phosphates), and solid polymers. These batteries are an emerging option for next-generation traction batteries promising low cost, high performance, and high safety. In 1965, the development of solid-state batteries was hindered by the lack of conductive ionic solids.
Solid-state batteries have found use in pacemakers, RFID, and wearable devices. They are potentially safer, with higher energy densities, but at a much higher cost. Due to higher electrochemical stability, high potential cathodes and even metallic Li may be used as anode leading to higher specific energy. Solid-state electrochemical energy storage devices such as all-solid-state batteries and supercapacitors have attracted huge attention to replacing the traditional aprotic liquid electrolyte based electrochemical energy storage systems. The desire for a portable electronic device has pushed the technological enhancements in rechargeable solid-state batteries. Solid-state electrolyte enables the integration of better-performed materials such as lithium metal and high-voltage cathode materials. However, it has been observed that the early-generation solid-state batteries may contain similar types of active electrode materials, with the liquid electrolyte being replaced by a solid-state electrolyte.
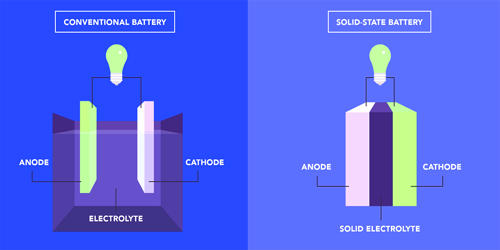
Fig: Solid-state Battery Transformation
Challenges to widespread adoption include energy and power density, durability, material costs, sensitivity, and stability. Most current lithium-ion technologies employ liquid electrolyte, with lithium salts such as LiPF6, LiBF4, or LiClO4 in an organic solvent. However, the solid electrolyte interface, which is caused as a result of the decomposition of the electrolyte at the negative electrode, limits the effective conductance. The development of solid-state batteries which would help in overcoming the main problems of batteries containing liquid electrolytes, i.e. leakage and/or corrosion at the electrodes, required the use of solid electrolytes with high ionic conductivity in order to limit the ohmic drop at the electrodes. Current high-end lithium-ion batteries can reach an energy density of over 700 Wh/L at the cell level, with a maximum driving range of about 500 Km for electric vehicles. The high-nickel-cathode materials being improved may further push the energy density but the characteristics of the active materials may draw a threshold.