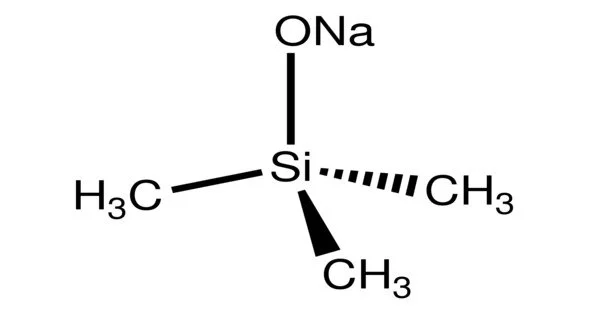
Sodium trimethylsiloxide is an organosilicon compound with the formula NaOSi(CH3)3. It is a salt composed of a sodium cation and a trimethylsilyloxide anion. It is the sodium salt of the conjugate base derived from trimethylsilanol. The compound is a strong base and a useful reagent in organic chemistry. It is a white solid with a molecular structure that consists of a cluster with Na-O-Na linkages based on closely related compounds.
Sodium trimethylsiloxide is a versatile reagent and has found applications in a variety of chemical reactions, such as in the formation of carbon-silicon bonds, the deprotonation of weakly acidic compounds, and the deoxygenation of sulfoxides to sulfides. The salt is used to prepare trimethylsiloxide complexes via salt metathesis. Trimethylsiloxide is a lipophilic pseudohalide. It is a source of oxide dianion.
Properties
Sodium trimethylsiloxide is a colorless to pale yellow liquid or solid, depending on the conditions of storage and use. It is highly reactive and should be handled with care. The compound can react violently with water and other protic solvents, so it should be stored and handled in a dry and inert atmosphere.
- Chemical formula: C3H9 NaOSi
- Molar mass: 112.179 g·mol−1
- Appearance: white solid
- Density: 1.255 g/cm3
- Melting point: 147–150 °C (297–302 °F; 420–423 K)
Application
Sodium trimethylsiloxide is commonly used as a deprotonating agent in organic synthesis. It is often used in reactions that involve the removal of acidic protons from substrates, such as in the preparation of enolates, which are important intermediates in organic synthesis. Sodium trimethylsiloxide is also used in the synthesis of organosilicon compounds, which have a wide range of industrial and technological applications.
One important use of sodium trimethylsiloxide is in organic synthesis as a mild base, often used to deprotonate alcohols or amides. It is also used as a deoxygenating agent, converting sulfoxides to sulfides. In addition, sodium trimethylsiloxide can be used as a nucleophile, reacting with a variety of electrophiles to form carbon-silicon bonds.
Safety Issue
However, sodium trimethylsiloxide is a highly reactive compound and should be handled with caution. It is moisture-sensitive and can react violently with water or protic solvents, releasing toxic gases. It can also react with air to form explosive peroxides, so it is important to store it under an inert gas atmosphere.