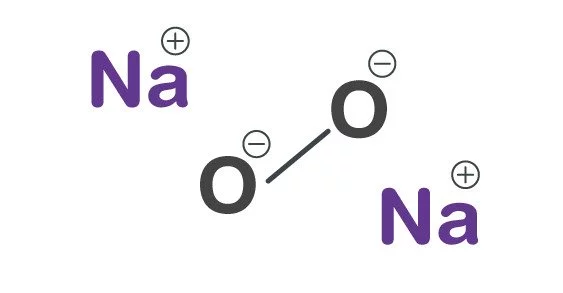
Sodium peroxide has the formula Na2O2 and is an inorganic compound. It has the appearance of a yellow-white to yellow granular solid. This yellowish solid is the result of sodium being ignited in an oxygen-rich environment. It’s a solid foundation. When this compound is mixed with any combustible material, it easily ignites due to heat, moisture contact, or friction.
Na2O2•2H2O2•4H2O, Na2O2•2H2O, Na2O2•2H2O2, and Na2O2•8H2O are all hydrates and peroxyhydrates of this metal peroxide. In contrast to the anhydrous material, the octahydrate, which is easily prepared, is white.
Properties
Sodium peroxide is a pale yellow solid, stable at ambient temperature, and hygroscopic. On heating, it starts to liberate oxygen at about 300 °C and decomposes rapidly above its melting point of 460 °C.
- Chemical formula: Na2O2
- Molar mass: 77.98 g/mol
- Appearance: yellow to white powder
- Density: 2.805 g/cm3
- Melting point: 460 °C (860 °F; 733 K) (decomposes)
- Boiling point: 657 °C (1,215 °F; 930 K) (decomposes)
- Solubility in water: Reacts
- Solubility: Soluble in acid
- Insoluble in base
- Reacts with ethanol
- Crystal structure: hexagonal
The hexagonal symmetry of sodium peroxide crystallizes. At 512°C, the hexagonal form undergoes a phase transition into an unknown symmetry. With additional heating above the boiling point of 657°C, the compound decomposes to Na2O, releasing O2.
2 Na2O2 → 2 Na2O + O2

Preparation
The octahydrate is produced by treating sodium hydroxide with hydrogen peroxide. Sodium peroxide can be prepared on a large scale by the reaction of metallic sodium with oxygen at 130–200 °C, a process that generates sodium oxide, which in a separate stage absorbs oxygen:
4 Na + O2 → 2 Na2O
2 Na2O + O → 2 Na2O2
It can also be made by passing ozone gas through a platinum or palladium tube containing solid sodium iodide. The sodium is oxidized by the ozone to form sodium peroxide. Iodine can be sublimed by gently heating it. The reaction is catalyzed by platinum or palladium, which is not attacked by sodium peroxide.
Uses
Sodium peroxide is used as an oxidizing agent and as an oxygen source by reacting with carbon dioxide to produce oxygen and sodium carbonate; it is thus especially useful in scuba gear, submarines, and other similar applications.
- It is used to bleach wood pulp.
- It is used in the production of paper and textiles.
- It is used for the extraction of minerals from various ores.
- It is used as an oxidizing agent.
- It is useful in scuba gear, and submarines.
- It is thus particularly useful in scuba gear, submarines, etc. Lithium peroxide and potassium superoxide have similar uses.