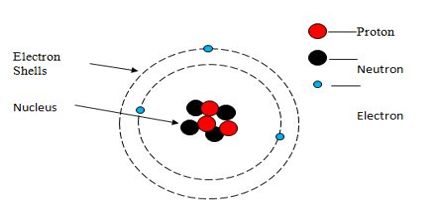
The aim of this lecture is to present on Simple Atomic Structure. Atoms are the foundation of chemistry. They are the basis for everything in the Universe. Electrons are the smallest of the three particles that make up atoms. Electrons are found in shells or orbitals that surround the nucleus of an atom. Protons and neutrons are found in the nucleus. Two positive protons and two negative electrons, Gain an electron to become negative and lose an electron to become positive. Here briefly focus on uses of Static Electricity.
Simple Atomic Structure