Evolutionary biologists at Johns Hopkins Medicine announce that they have coupled PET scans of modern pigeons with dinosaur fossil studies to help answer an ongoing mystery in biology. How did birds’ brains evolve so they could fly?
According to their findings, the explanation appears to be an adaptive increase in cerebellar size in some fossil vertebrates. The cerebellum is the part of the brain that controls movement and motor function. The research findings have been published in the Proceedings of the Royal Society B.
Scientists have long thought that the cerebellum should be important in bird flight, but they lacked direct evidence. To pinpoint its value, the new research combined modern PET scan imaging data of ordinary pigeons with the fossil record, examining brain regions of birds during flight and braincases of ancient dinosaurs.
Powered flight among vertebrates is a rare event in evolutionary history. Only three groups of vertebrates, or animals with a backbone, evolved to fly: extinct pterosaurs, the terrors of the sky during the Mesozoic period, which ended over 65 million years ago; bats; and birds.
Amy Balanoff
“Powered flight among vertebrates is a rare event in evolutionary history,” says Amy Balanoff, Ph.D., assistant professor of functional anatomy and evolution at the Johns Hopkins University School of Medicine and first author on the published research.
According to Balanoff, only three groups of vertebrates, or animals with a backbone, evolved to fly: extinct pterosaurs, the terrors of the sky during the Mesozoic period, which ended over 65 million years ago; bats; and birds. The three species are not closely related on the evolutionary tree, and the crucial factors or variables that allowed flight in all three have remained unknown.
Aside from obvious physical adaptations for flight, such as extended upper limbs, specific types of feathers, a streamlined body, and other traits, Balanoff and her colleagues designed studies to identify features that resulted in a flight-ready brain. To accomplish this, she collaborated with biomedical engineers at Stony Brook University in New York to examine the brain activity of modern pigeons before and after flight.
The researchers utilized positron emission tomography, or PET, imaging scans, the same technology used on people, to compare activity in 26 brain regions when the bird was at rest and immediately after it flew for 10 minutes from one perch to another. They scanned eight birds on various days.
PET scans employ a substance comparable to glucose that can be tracked to the location where brain cells absorb it the greatest, suggesting higher energy use and consequently activity. The tracker degrades and is expelled by the body after a day or two.
Of the 26 regions, one area – the cerebellum – had statistically significant increases in activity levels between resting and flying in all eight birds. Overall, the level of activity increase in the cerebellum differed by more than two standard statistical deviations, compared with other areas of the brain.
The researchers also detected increased brain activity in the so-called optic flow pathways, a network of brain cells that connect the retina in the eye to the cerebellum. These pathways process movement across the visual field. Balanoff says their findings of activity increase in the cerebellum and optic flow pathways weren’t necessarily surprising, since the areas have been hypothesized to play a role in flight.
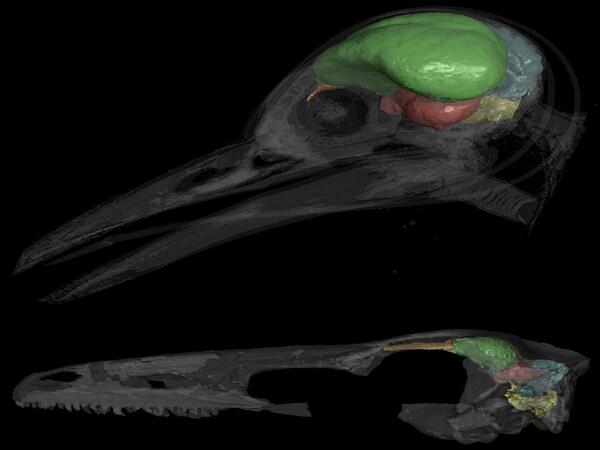
What was novel about their research was connecting the cerebellar discoveries of flight-enabled brains in current birds to the ancient record, which revealed how the brains of birdlike dinosaurs evolved brain conditions for powered flight. To do this, Balanoff utilized a digital database of endocasts, or molds of the inside space of dinosaur skulls that, when filled, mimic the brain.
Balanoff discovered and traced a significant rise in cerebellum volume to some of the first species of maniraptoran dinosaurs, predating the first indications of powered flight among early bird relatives, including Archaeopteryx, a winged dinosaur. Balanoff and her team also discovered evidence in the endocasts of increased tissue folding in the cerebellum of early maniraptorans, indicating increased brain complexity.
The researchers warned that these are preliminary data, and variations in brain activity during powered flight may also occur during other behaviors, such as gliding. They also point out that their studies comprised simple flying, with no obstructions and a clear flight path, and that other brain regions may be more engaged during complex flight maneuvers.
The next step for the research team is to identify specific parts of the cerebellum that permit a flight-ready brain, as well as the neural connections between these components. According to Gabriel Bever, Ph.D., associate professor of functional anatomy and evolution at the Johns Hopkins University School of Medicine, scientific theories for why the brain grows in size over evolutionary time include the need to traverse new and different landscapes, laying the groundwork for flight and other locomotive styles.