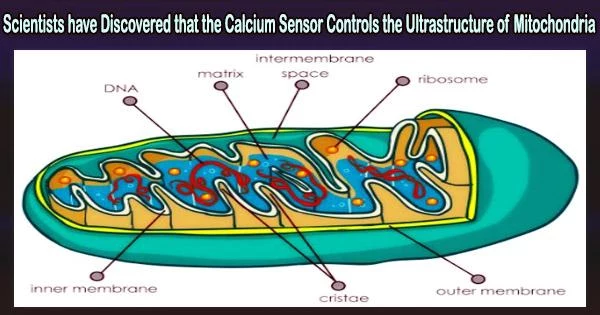
Mitochondria are often referred to as the “powerhouses” of the cell because they are responsible for generating most of the cell’s energy in the form of adenosine triphosphate (ATP). These organelles are found in the cytoplasm of eukaryotic cells, which include cells of plants, animals, and fungi.
Cell signaling and function depend on calcium homeostasis, which involves calcium ion movement within cells. Importantly, calcium fine-tunes energy generation in the mitochondria, the cellular powerhouses.
According to recent findings from the Lewis Katz School of Medicine at Temple University, calcium plays a crucial role in the regulation of mitochondrial ultrastructure in addition to its other important roles in the mitochondria.
The new research, published in the journal Science Signaling, is the first to show the calcium-binding protein MICU1 regulates that mitochondrial ultrastructure.
The mitochondrial contact site and cristae organizing system (MICOS), which controls the composition of the inner and outer mitochondrial membranes, depends on MICU1 to function. For controlling cellular energetics and cell death, calcium, MICU1, and MICOS must work together in a carefully planned manner.
“We knew from previous work that MICU1 binds and regulates MCU, the main calcium transporter in mitochondria, and that deletion of the MICU1 gene disrupts mitochondrial structure,” explained John W. Elrod, Ph.D., Director of the Cardiovascular Research Center at the Katz School of Medicine and senior investigator on the new study.
“Dhanendra Tomar, first author on the study, discovered that when MCU is deleted, MICU1 still forms high-molecular weight complexes. This suggested to us that MICU1 was binding and doing something other than regulating calcium uptake through the MCU channel.”
MICOS is primarily responsible for dictating the structure of the inner and outer mitochondrial membranes, including the folding of the inner membrane into cristae.
Dr. John W. Elrod
Using a technique called proximity biotinylation, Dr. Elrod and associates fused biotin ligase to MICU1 protein molecules in order to look into the possibility of MICU1 serving a second purpose in mitochondria. Any protein that interacted closely with MICU1 in this system became biotin-labeled.
After that, the study team generated the MICU1-biotin ligase fusion protein in cells deficient in MCU in order to separate and pinpoint MICU1 binding partners that were unrelated to its conventional function of interfacing with the mitochondrial calcium uniporter channel.
Surprisingly, the researchers discovered that MICU1 and two MICOS complex components interacted extensively.
“MICOS is primarily responsible for dictating the structure of the inner and outer mitochondrial membranes, including the folding of the inner membrane into cristae,” Dr. Elrod said.
Cristae play a significant part in controlling the electron transport chain, bioenergetics, and cell death, among other mitochondrial processes.
The research offers a conceptual framework for comprehending calcium signaling at the mitochondrial membrane as opposed to the interior region, or matrix, of the mitochondria. The new discoveries also shed light on the cell signaling networks behind mitochondrial remodeling, a characteristic of many diseases.
“Numerous diseases are impacted by mitochondrial ultrastructure, and some MICOS components are altered in cardiovascular disease, such as heart failure,” Dr. Elrod added.
Although it will be a while before knowledge of MICU1 and MICOS can be used to create new treatments, understanding how these two proteins interact is crucial for drug discovery.