Cellulose nanocrystals (CNCs) are a type of nanomaterial that can be extracted from cellulose, which is the most abundant natural polymer on earth and the main component of plant cell walls.
The usage of cellulose nanocrystals in water treatment, packaging, tissue engineering, electronics, antibacterial coatings, and many other applications makes them important bio-based nanomaterials.
Despite the fact that the materials offer a sustainable alternative to non-biobased products, transporting them in liquid strains industrial infrastructure and has an adverse effect on the environment.
CNCs have a number of desirable properties that make them attractive for a range of applications, including their high mechanical strength, stiffness, and aspect ratio (i.e., length-to-width ratio), as well as their biodegradability, renewability, and low toxicity.
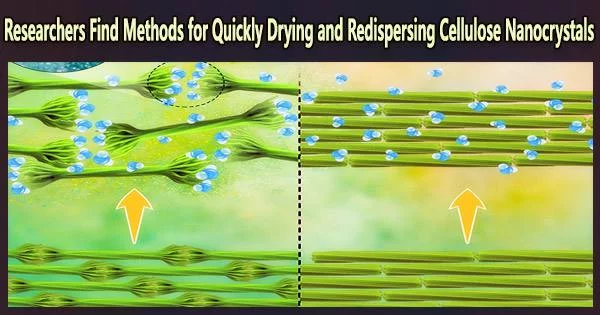
In order to make nanocrystals easier to store and transport, a group of chemical engineering researchers at Penn State investigated the mechanisms of drying them. They then proposed using nanotechnology to make the nanocrystals highly redispersible in aqueous mediums while maintaining their full functionality. They published their results in the journal Biomacromolecules. The work also will be featured on the Jan. 17 journal cover.
The hairy nanocrystals can become redispersed even at high salt concentrations, which is convenient, as they remain functional in harsh media and may be used in a broad range of applications. This work may pave the way for sustainable and large-scale processing of nanocelluloses without using additive or energy-intensive methods.
Mica Pitcher
“We looked at how we could take hairy nanocrystals, dry them in ovens, and redisperse them in solutions containing different ions,” said co-first author Breanna Huntington, current chemical engineering doctoral student at the University of Delaware and former member of the Sheikhi Research Group while an undergraduate student at Penn State. “We then compared their functionality to conventional, non-hairy cellulose nanocrystals.”
The hair-like, negatively charged cellulose chains on the nanocrystals’ ends. The phrase electrosteric repulsion, which means charge-driven, or electrostatic, and free-volume dependant, or steric, means that when the hairs are rehydrated, they repel one another and separate, dispersing once more across a liquid.
“The hairy ends of the nanocrystals are nanoengineered to be negatively charged and repel each other when placed in an aqueous medium,” said corresponding author Amir Sheikhi, Penn State assistant professor of chemical engineering and of biomedical engineering. “To have maximum function, the nanocrystals must be separate, individual particles, not chained together as they are when they are dry.”
Researchers analyzed and assessed the size and surface qualities of the redispersed hairy particles and discovered that their features and performance were identical to those of the undried particles. The particles performed well and maintained their stability in a variety of liquid mixes with varying salinities and pH levels, the researchers discovered.
“The hairy nanocrystals can become redispersed even at high salt concentrations, which is convenient, as they remain functional in harsh media and may be used in a broad range of applications,” said co-first author Mica Pitcher, Penn State doctoral student in chemistry, supervised by Sheikhi. “This work may pave the way for sustainable and large-scale processing of nanocelluloses without using additive or energy-intensive methods.”
The Penn State College of Engineering Summer Research Experiences for Undergraduates program and the NASA Pennsylvania Space Grant Consortium graduate fellowship program supported this work.