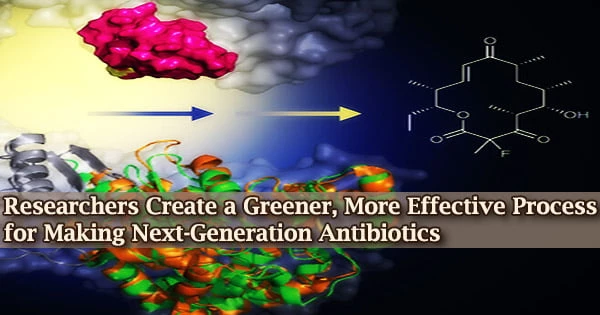
A technique for modifying one class of antibiotics has been devised by an international team of researchers using microscopic organisms that naturally manufacture these chemicals. The research, which was released on July 25, 2022, in Nature Chemistry, might make it possible to produce medicines that are efficient against drug-resistant microorganisms.
A microbe that is genetically preprogrammed to manufacture the antibiotic erythromycin was the team’s starting point.
Researchers from Germany’s Goethe University’s Institute of Organic Chemistry and Chemical Biology wondered if the system could be genetically changed to build the antibiotic with one more fluorine atom, which is frequently used to boost medicinal qualities.
“We had been analyzing fatty acid synthesis for several years when we identified a part of a mouse protein that we believed could be used for directed biosynthesis of these modified antibiotics if added to a biological system that can already make the native compound,” said Martin Grininger, professor for biomolecular chemistry at Goethe University.
The scientists employed protein engineering to replace a portion of the system’s natural machinery with the functionally equivalent mouse gene in collaboration with David Sherman’s group at the University of Michigan, which specializes in this biological assembly system.
“It’s like taking one engine part out of a Mercedes and putting it into a Porsche to make a better hybrid engine. You get a Porsche engine that can do new things and works even better,” said Sherman, a faculty member at the U-M Life Sciences Institute and professor of medicinal chemistry in the College of Pharmacy.
“We can now take advantage of this protein engineering to make new compounds that have this very desirable fluorine atom, which chemists have been struggling to add to macrolide antibiotics for a long time.”
The addition of this fluorine atom makes the final product more appealing since it alters both its ultimate structure and its capacity to safely treat patients while also killing microorganisms. Erythromycin inhibits the action of the bacterial ribosome, which is necessary for bacteria to thrive, by attaching to it and binding to it.
We had been analyzing fatty acid synthesis for several years when we identified a part of a mouse protein that we believed could be used for directed biosynthesis of these modified antibiotics if added to a biological system that can already make the native compound.
Professor Martin Grininger
Some bacteria have developed strategies to avoid this binding, making them immune to antibiotic therapy. By adding a fluorine atom to the antibiotic, this evolutionary advantage is removed, restoring the compound’s capacity to combat bacteria.
Although scientists have devised techniques for synthetically adding fluorine, the procedure is laborious and necessitates the use of hazardous chemical reagents. These issues are resolved by the novel biosynthetic technique created by researchers from Goethe University and the University of Michigan.
“It’s a very exciting development because we can bypass all the time-consuming synthetic steps and dangerous chemicals,” Sherman said. “We have shown that we can basically reprogram an organism to make the fluorinated product directly.”
The availability of fluorinated chemicals in clinical settings is still a few years off, according to the researchers. However, the discoveries suggest a more effective course for creating new antibiotics, as well as antivirals and anti-cancer drugs.
“Our approach has been proven successful on a small set of antibiotics, but it could ultimately be used to develop a wide range of pharmaceuticals with minimal use of toxic chemicals and by-products,” Grininger said.
The National Institutes of Health, the LOEWE program of the state of Hesse, and the Volkswagen Foundation all provided funding for this study.
Other study authors are: Alexander Rittner, Mirko Joppe, Lara Maria Mayer, Simon Reiners, Elia Heid and Dietmar Herzberg of the Buchmann Institute for Molecular Life Sciences, Goethe University Frankfurt, Germany; and Jennifer Schmidt of the University of Michigan Life Sciences Institute.