By enabling the rapid study of multiple therapeutic combinations against tumor cells, an innovative testing platform that more closely mimics what cancer encounters in the body may allow for more precise, personalized therapies. The platform, which uses a three-dimensional environment to mimic a tumor microenvironment, is demonstrated in a study published in Communications Biology.
“This entire platform really gives us a way to optimize personalized immunotherapy on a rapid, high throughput scale,” said Jonathan Dordick, Institute Professor of chemical and biological engineering and member of Rensselaer Polytechnic Institute’s Center for Biotechnology and Interdisciplinary Studies (CBIS), who led this research. “Imagine someone has cancer, and you quickly biopsy the tumor, and then you use this biochip platform to identify very quickly — within a day or two — what specific treatment modality might be ideally suited against specific cancer.”
An innovative testing platform that more closely mimics what cancer encounters in the body may allow for more precise, personalized therapies by enabling the rapid study of multiple therapeutic combinations against tumor cells.
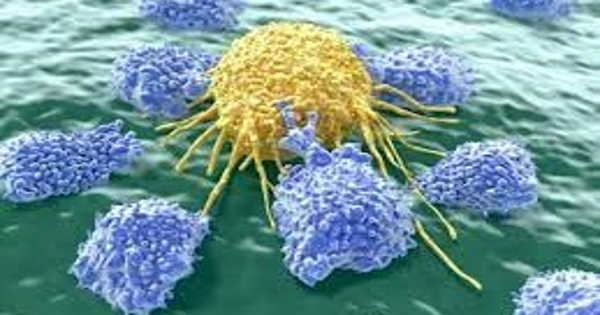
The behavior of a specific type of immune cell known as natural killer (NK) cells, which seek out cancer or viruses within the body, bind to their receptors, and excrete an enzyme meant to kill the unwanted cells, is of particular interest to researchers. The platform investigated in this paper enables researchers to compare what happens when NK cells are left to fight tumor cells on their own versus how they behave when an antibody, cancer drug, or a combination of the two is added.
The platform consists of a two-piece plastic chip about the size of a microscope slide. On one side of the sandwich chip, researchers can place an external matrix made of a gel-like substance that mimics the mechanical environment of a tumor cell. Cancer cells are encouraged to grow into a spheroid shape when placed inside this gel-like structure, just as they would in the body. The second component includes 330 microwells into which NK cells can be added in suspension, much like they would flow untethered inside the body.
Dordick worked at Rensselaer with Seok-Joon Kwon, a senior research scientist in CBIS, and Sneha Gopal, who recently received her Ph.D. in part due to this research. Rensselaer researchers collaborated with Konyang University and Medical & Bio Decision Company Ltd. To put this platform to the test, researchers used different combinations of NK cells, two monoclonal antibodies, and an anti-cancer chemotherapy drug on two types of breast cancer cells and pancreatic cancer cells.
“You can quickly screen to see what combinations of NK cells, antibodies, and chemotherapeutic drugs target the cancer cells within the spheroid geometry,” Dordick said. “What’s really amazing is that we see very significant differences between what happens in that spheroid, within the slots of the chip, and what happens in a more traditional two-dimensional cell culture, which is often used in screening.”
The chemotherapy drug paclitaxel, for example, had little effect on the three types of cancer cells on its own in the spheroid design, whereas the drug may appear to do well in a traditional two-dimensional system, according to Dordick. When combined with both NK cells and an antibody, it performed significantly better.
“This platform brings researchers closer to personalized medicine,” said CBIS director Deepak Vashishth. “This work by Professor Dordick and his research group is an excellent example of how, at Rensselaer, we are bringing a new perspective to human health by developing new approaches at the intersection of engineering and life sciences to improve cures for diseases like cancer.”
Dordick stated that this tool must be tested on a wide range of cancer types, including a tumor microenvironment composed of multiple different types of cells, to further its potential use. He hopes that in the future, the platform will be able to identify combination therapies that work best against a patient’s specific cancer, allowing for the identification and delivery of personalized immunotherapy.