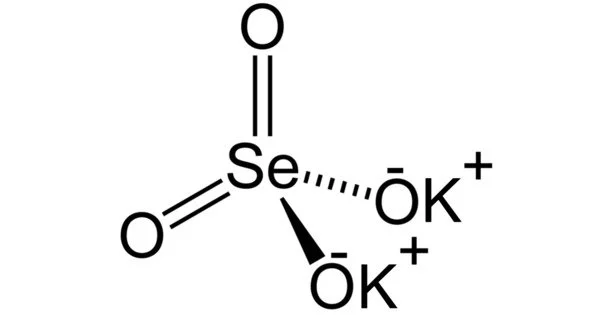
Potassium selenate, K2SeO4, is an odorless, white solid that forms as the potassium salt of selenic acid. It is relatively stable under normal conditions. It does not react vigorously with water or air. However, it may react with reducing agents or other reactive chemicals. It can be used as a source of selenium in fertilizers to supplement selenium-deficient soils. It is also utilized in the production of glass and ceramics.
Properties
- Molecular Weight: 221.22 g/mol
- Appearance: It is a white crystalline solid.
- Solubility: It is highly soluble in water.
Preparation
Potassium selenate can be prepared by reacting selenic acid (H2SeO4) with potassium hydroxide (KOH) or potassium carbonate (K2CO3). It is produced by the reaction of selenium trioxide and potassium hydroxide.
SeO3 + 2 KOH → K2SeO4 + H2O
Alternatively, it can be made by treating selenous acid with potassium hydroxide, followed by oxidation of the resulting potassium selenite with bromine water.
H2SeO3 + 2 KOH → K2SeO3 + 2 H2O
K2SeO3 + 2 KOH + Br2 → K2SeO4 + 2 KBr + H2O
Uses
Potassium selenate can be used to produce selenium trioxide. It can also use to treat selenium deficiency in livestock. It can be used as a source of selenium, an essential micronutrient for plant growth. It is sometimes added to fertilizers to provide selenium to crops. It is utilized in the production of other selenium compounds, such as sodium selenate and selenium dioxide.
It can be used as a reagent or standard in analytical methods to determine selenium content in samples. It is sometimes used in laboratory experiments or as a supplement in animal studies to investigate the effects of selenium.
Safety
Potassium selenate is generally considered to be low in toxicity. However, like other selenium compounds, it should be handled with care as high concentrations can be harmful.
Environmental Impact
Potassium selenate can pose a risk to aquatic organisms if released into water bodies in large quantities. Proper disposal and adherence to environmental regulations are important.