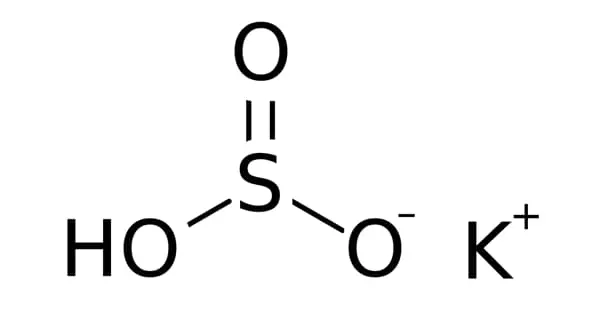
Potassium bisulfite is a chemical compound with the chemical formula KHSO3. It is a potassium salt that is inorganic. When heated from 300 to 700°C, it transforms to molten form, and the reactions that occur during this transition have been studied using Raman spectra. It is used to preserve colorless foods such as fruit juices, squashes, apples, and raw mango chutney.
In reality, potassium bisulfite is a salt mixture that dissolves in water to form solutions containing potassium ions and bisulfite ions. It is a white solid with a sulfur dioxide odor. Attempts to crystallize potassium bisulfite result in the formation of potassium metabisulfite, K2S2O5.
Properties
Potassium bisulfite solution is a colorless aqueous solution with a mild sulfur dioxide odor. It is a water-soluble inorganic salt that is primarily used as a preservative, a flux, and in the production of fertilizers. It serves as a reducing agent as well as a chemical preservative.
- Molecular Weight: 136.16
- Appearance: White crystals, granules, or powder
- Melting Point: 195-197 °C
- Boiling Point: N/A
- Density: 2.24 g/cm3
- Solubility in H2O: N/A
- Exact Mass: 135.923261

Production
Sulfur dioxide and potassium carbonate react to form it. The sulfur dioxide is passed through a potassium carbonate solution until no more carbon dioxide is produced. The solution has a high concentration.
When used in accordance with good manufacturing or feeding practices, potassium bisulfite is generally recognized as safe by the FDA, with the exception that it is not used in meats or foods recognized as sources of vitamin B1.
Application
In the production of alcoholic beverages, potassium bisulfite is used as a sterilizing agent. Under the current EU-approved food additive legislation, this additive is classified as E number E228.
Potassium bisulfate may be used:
- As a catalyst for the synthesis of butyl paraben from p-hydroxybenzoic acid and n-butyl alcohol.
- As a promoter for the synthesis of 3,4-dihydropyrimidin-2(1H)-one derivatives in ethylene glycol via the Biginelli reaction.
- As an acid catalyst in the reactive distillation of ethyl tert-butyl ether (ETBE) from ethanol and tert-butyl alcohol.
- As a dehydrating agent in the synthesis of pyruvic acid from tartaric acid.
Butyl paraben and methyl cinnamate can be synthesized using potassium bisulfate as a catalyst. It can also be used to calculate the amount of beryllium in beryl using gravimetry.
Toxicity
Potassium bisulfate is a white crystalline solid with a sulfur odor. Ingestion of potassium bisulfate may result in illness. Potassium bisulfate may emit toxic fumes when heated to high temperatures.