Ocean acidification is primarily the result of enormous amounts of CO2 being released into the atmosphere by automobiles, industrial, and agricultural operations. Chemical processes occur when carbon dioxide (CO2) is absorbed by saltwater, lowering the pH, carbonate ion concentration, and saturation states of biologically essential calcium carbonate minerals. ‘Ocean acidification,’ or ‘OA’ for short, is the name given to these chemical processes.
The combustion of fossil fuels is the primary cause of ocean acidification. One of the many consequences of climate change on the seas is ocean acidification. Since the beginning of the Industrial Revolution about 1750, the seas have absorbed around one-third to one-half of the CO2 emitted into the atmosphere by human activity. According to experts, the average pH of seawater fell from 8.19 to 8.05 over that time period, corresponding to a 30 percent rise in acidity.
About 30% of the carbon dioxide (CO2) emitted into the atmosphere is absorbed by the ocean. The quantity of carbon dioxide absorbed by the ocean increases when atmospheric CO2 levels rise due to human activities such as burning fossil fuels (e.g., automobile emissions) and altering land use (e.g., deforestation). Ocean acidification involves a move towards pH-neutral circumstances rather than a transition to acidic conditions. Seawater is somewhat basic (meaning pH > 7), and ocean acidification involves a shift towards pH-neutral conditions rather than a transition to acidic conditions (pH < 7).

Ocean acidification is a source of worry since it can lead to a reduction in the formation of calcium carbonate shells in shellfish and other aquatic life, as well as other physiological problems for marine creatures. According to some experts, the rate of ocean acidification has been 100 times faster since the beginning of the Industrial Revolution than at any previous time in the last 650,000 years. Between 1000 and 1900 CE, atmospheric CO2 concentrations varied between 275 and 290 parts per million by volume (ppmv), according to the researchers.
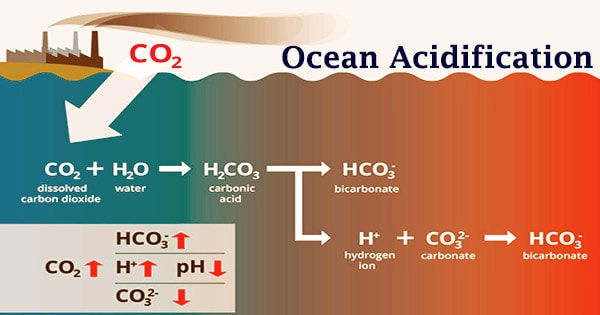
The pH of seawater is frequently used to describe ocean acidification. The pH scale measures acidity and alkalinity. A pH of less than 7 is acidic, whereas a pH of higher than 7 is alkaline, or basic. The pH value of the ocean surface is estimated to have decreased from approximately 8.25 to 8.14 between 1751 and 1996, representing an increase of nearly 30% in H+ ion concentration in the world’s oceans (note that the pH scale is logarithmic, so a change of one pH unit equals a ten fold change in H+ ion concentration).
Because the pH scale is inversely proportional to hydrogen ion concentration, more hydrogen ions equals more acidity and a lower pH. Continued ocean acidification, on the other hand, is leading many regions of the ocean to become undersaturated with these minerals, potentially affecting certain species’ capacity to create and maintain shells. With more CO2 in the seas, pH would drop even further; worst-case scenarios would see seawater pH plummet to between 7.8 and 7.9 by 2100.
However, when ocean acidification worsens, available carbonate ions (CO32-) bind with excess hydrogen, leaving calcifying organisms with fewer carbonate ions to create and maintain their shells, skeletons, and other calcium carbonate structures. Shells and skeletons may begin to disintegrate if the pH falls too low. According to projections of future carbon dioxide levels based on business-as-usual emission scenarios, the ocean’s surface waters may be about 150 percent more acidic by the end of the century, culminating in a pH that the oceans haven’t seen in more than 20 million years.
Water acidification, according to marine experts, poses a threat to sea life and societies that rely on the ocean for food and livelihood. Increased acidity is considered to have a variety of potentially detrimental effects on marine species, including lowering metabolic rates and immunological responses in some organisms, as well as triggering coral bleaching. Increases in ocean acidity lower carbonate ion concentrations and aragonite (a major source of calcium carbonate) availability in saltwater.
Non-calcifying species can also be affected by changes in ocean chemistry. In increasingly acidic environments, some fish, such as clownfish, lose their capacity to detect predators. The pH of saltwater nowadays is extremely changeable, and a single organism may adapt to a wide range of pH values over the course of its existence. The issue with ocean acidification is that it is a long-term phenomenon, with the risk stemming from lifetime exposure to decreasing pH levels.
Coral, shellfish, and other marine calcifiers (organisms that consume carbonates) are expected to have a harder time obtaining the basic materials they need to construct and maintain their skeletons and shells, according to marine experts. Reduced pH levels have been demonstrated to impact the capacity of larval clownfish link to find appropriate habitat in studies.
When these creatures are threatened, the entire food web may be threatened as well. Ocean acidification is predicted to have varied effects on ocean organisms. Higher CO2 levels in the water may help photosynthetic algae and seagrasses, as they, like plants on land, require CO2.
Both organic and inorganic carbon molecules, such as carbon dioxide, carbonate ion, and bicarbonate ion, are involved in the carbon cycle. Lower ambient calcium carbonate saturation levels, on the other hand, have been found to have a dramatic influence on some calcifying organisms, such as oysters, clams, sea urchins, shallow water corals, deep-sea corals, and calcareous plankton, according to research. More than a billion people throughout the world rely on seafood as their major source of nutrition.
Changes in marine ecosystems will have ramifications for human cultures that rely on the commodities and services provided by these ecosystems. Significant income losses, loss of jobs and livelihoods, and other indirect economic expenses might all be consequences for society. One element of global climate change is ocean acidification. Anything we do now to combat climate change will help the ocean’s future as well.
Information Sources: