History:
The Arndt-Eistert synthesis is a series of chemical reactions designed to convert a carboxylic acid to a higher homologue.( i.e. contain one additional carbon atom ) and is considered a homologation process. Named for German chemists Fritz Arndt (1885-1969), and Bernd Eistert (1902-1978)
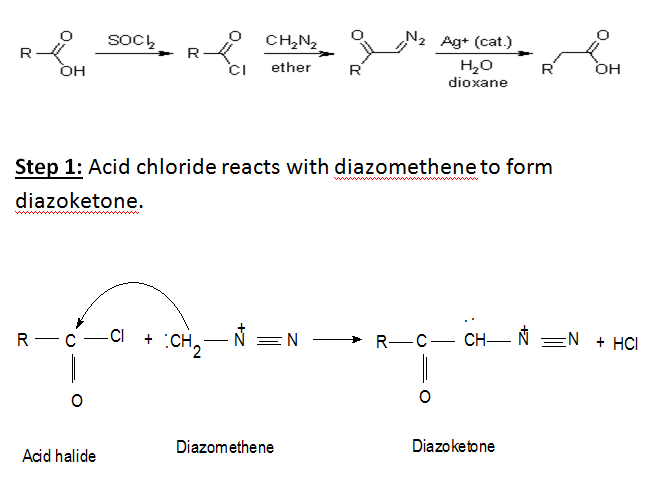
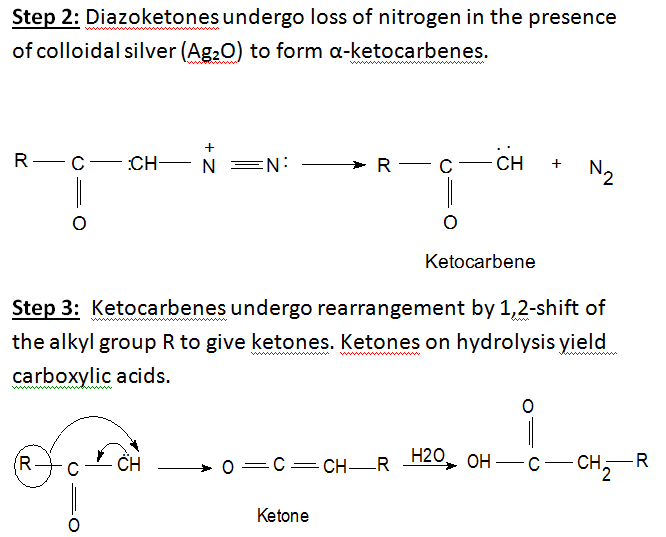
Basic method of reaction: Arndt-Eistert synthesis is a popular method of producing B(beta)-amino acid from @(alpha)-amino acid, Acid chlorides react with diazomethane to give diazoketones.In the presence of a nucleophile (water) and a metal catalyst (silver oxides) diazoketones will form the desired acid homologue.
Principle:
The Arndt-Eistert Synthesis allows the formation of homologated carboxylic acids or their derivatives by reaction of the activated carboxylic acids with diazomethane and subsequent Wolff-Rearrangement of the intermediate diazoketones in the presence of nucleophiles such as water, alcohols, or amines.
Application:
- By Arndt-Eistert reaction we can synthesise propionic acid from acetic acid.
Abstract :
- The Arndt–Eistert reaction offers a convenient method for the synthesis of the higher members of the aliphatic carboxylic acid series. Nonadecanoic acid, eicosanoic acid, and heneicosanoic acid have been prepared successively from stearic acid in good yields. An efficient method of purification of the synthetic products is described. The ultraviolet absorption maxima for some diazoketones derived from the higher members of the aliphatic carboxylic acid series are recorded.
- The chain extension of carboxylic acids developed by Arndt and Eistert was applied to the construction of peptides containing β-amino acids. The method may be used for the generation of new types of peptide-containing combinatorial libraries.
Reference:
- ^ Arndt, F.; Eistert, B. (1935). “Ein Verfahren zur Überführung von Carbonsäuren in ihre höheren Homologen bzw. deren Derivate” (in German). Ber. Dtsch. Chem. Ges. 68 (1): 200–208. doi:10.1002/cber.19350680142.
- ^ Bachmann, W. E.; Struve, W. S. (1942). “The Arndt-Eistert Reaction”. Org. React. 1: 38.
- ^ Ye, T.; McKervey, M. A. (1994). “Organic Synthesis with α-Diazo Carbonyl Compounds”. Chem. Rev. 94 (4): 1091–1160. doi:10.1021/cr00028a010.
- ^ Lee, V.; Newman, M. S. (1970), “Ethyl 1-Naphthylacetate”, Org. Synth. 50: 77; Coll. Vol. 6: 6