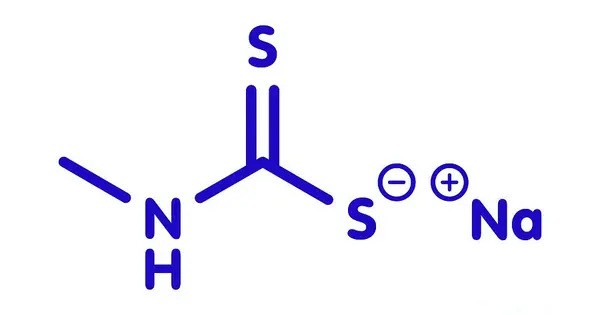
Metam sodium is an organosulfur compound with the formula CH3NHCS2Na. The compound is a sodium salt of a dithiocarbamate. It is a chemical compound primarily used as a soil fumigant, pesticide, herbicide, and fungicide in agriculture. Its chemical formula is C₂H₄NNaS₂, and it’s often applied to soil before planting crops to control pests like nematodes, fungi, and weeds.
The compound exists as a colorless dihydrate, but most commonly it is encountered as an aqueous solution. It is used as a soil fumigant, pesticide, herbicide, and fungicide. It is one of the most widely used pesticides in the United States, with approximately 60 million pounds used in 2001.
Properties
- Chemical formula: C2H4NNaS2
- Molar mass: 129.18 g/mol
- Appearance: Yellow to brown liquid (usually in aqueous solution)
- Odor: Pungent, sulfur-like smell
- Solubility: Highly soluble in water
- pH (1% solution): ~10–12 (alkaline)
- Decomposition: Rapidly breaks down into methyl isothiocyanate (MITC), which is the active toxic agent.
- Boiling point: Decomposes before boiling
- Melting point: Not applicable (used in solution)
- Density: Approximately 1.15 g/cm³ (for 42% solution)
- Stability: Stable in alkaline solution but decomposes in acidic environments or when exposed to heat, moisture, or sunlight.
Preparation
Metam sodium is prepared by combining methylamine, carbon disulfide, and sodium hydroxide:
CH3NH2 + CS2 + NaOH → CH3NHCS2Na + H2O
It also arises from the reaction of methyl isothiocyanate and sodium thiolate.
Upon exposure to the environment, metam sodium decomposes to methyl isothiocyanate and other sulfur compounds.
Toxicology
- Toxic if inhaled, ingested, or in contact with skin.
- Decomposition product MITC is hazardous and associated with respiratory irritation and potential environmental harm.
- Classified as moderately hazardous by the WHO.
Application in Agriculture
- Commonly used to sterilize soil before planting high-value crops such as potatoes, tomatoes, strawberries, and carrots.
- Applied by injection into soil or via drip irrigation systems.
Breakdown in the Environment
- Rapidly converts into MITC after application.
- MITC can volatilize into the atmosphere, affecting nearby areas.
- Does not persist long in the environment but can pose risks during and shortly after application.