Mercury sulfide, commonly known as cinnabar, is a chemical compound composed of the chemical elements mercury and sulfur. Cinnabar typically appears as a deep red or crimson color. It is represented by the chemical formula HgS. It is virtually insoluble in water. It has a crystalline structure, often forming in tabular or granular forms. Mercury sulfide is insoluble in water and most acids.
Properties
It is typically a bright red to dark red mineral, though it can also appear in shades of black. It crystallizes in the hexagonal system, often forming as prismatic or tabular crystals. It is insoluble in water, but can dissolve in strong acids. It is toxic due to the mercury content, necessitating careful handling.
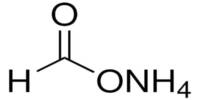
- Chemical formula: HgS
- Molar mass: 232.66 g/mol
- Density: 8.10 g/cm3
- Melting point: 580 °C (1,076 °F; 853 K) decomposes
- Solubility in water: insoluble
- Band gap: 2.1 eV (direct, α-HgS)
Occurrences
- Geological Settings: Commonly found in volcanic regions and hydrothermal veins. It often forms in hot springs and fumarolic deposits.
- Associated Minerals: Often found alongside minerals like quartz, calcite, and pyrite.
- Global Distribution: Notable deposits are located in Spain, Italy, China, and the USA (notably in California).
Uses
- Mercury Production: It is primarily used to extract mercury through roasting.
- Pigment: Cinnabar has historically been used as a pigment in art and decoration, known for its vivid red color.
Safety
Both mercury and its compounds are highly toxic. Proper precautions must be taken when handling mercury sulfide to avoid health risks.
Occurrence
Cinnabar is found in various geological settings, often in volcanic environments and hot spring deposits. The extraction and use of mercury sulfide can lead to environmental contamination due to the toxicity of mercury. Consequently, its mining and use are heavily regulated in many regions to mitigate health risks.