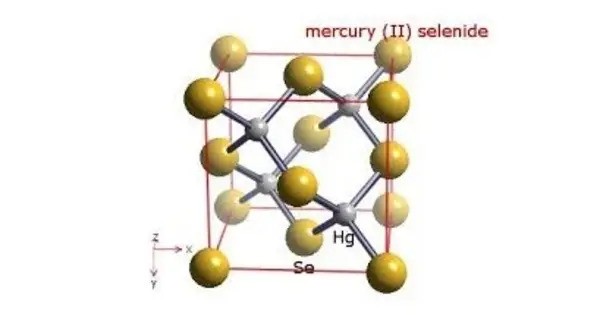
Mercury selenide is a chemical compound of mercury and selenium. It is a grey-black crystalline solid semi-metal with a sphalerite structure. The lattice constant is 0.608 nm. It is primarily known for its semiconductor properties and is often used in infrared detectors and other electronic applications.
HgSe has a cubic crystal structure and can form both n-type and p-type semiconductors depending on the doping elements used. HgSe occurs naturally as the mineral Tiemannite, and is a component of the “intimate mixture” of HgSe and Se known as HgSe2.
Along with other II-VI compounds, colloidal nanocrystals of HgSe can be formed. It has notable optical properties, including sensitivity in the infrared range, making it useful in photodetectors.
Properties
- Chemical formula: HgSe
- Molar mass: 279.55 g/mol
- Appearance: grey-black solid
- Odor: odorless
- Density: 8.266 g/cm3
- Melting point: 1,000 °C; 1,830 °F; 1,270 K
- Solubility in water: insoluble
- Crystal structure: sphalerite
Applications
Selenium is used in filters in some steel plants to remove mercury from exhaust gases. The solid product formed is HgSe. Besides its use in infrared sensors, HgSe is studied for potential applications in solar cells and other electronic devices.
HgSe can be used as an ohmic contact to wide-gap II-VI semiconductors such as zinc selenide or zinc oxide.
Toxicity
Toxic hydrogen selenide fumes can be evolved on exposure to acids. HgSe is non-toxic as long as it is not ingested due to its insolubility. Both mercury and selenium are toxic elements, so handling HgSe requires strict safety precautions to avoid exposure.