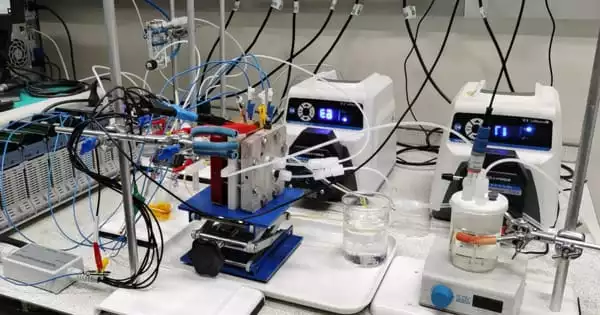
According to Imperial College London experts, a novel approach to battery architecture could hold the secret to low-cost, long-term energy storage. Engineers and scientists collaborated to develop a polysulfide-air redox flow battery (PSA RFB) with not one, but two membranes. The dual membrane design overcomes the primary issues with this sort of large-scale battery, allowing it to store extra energy from sources such as wind and solar. The findings were published in Nature Communications.
Energy is stored in liquid electrolytes that flow through the cells during charge and discharge, allowing for chemical reactions. The volume of the electrolyte determines the quantity of energy stored, making the system potentially straightforward to scale up. However, the electrolyte used in typical redox flow batteries, vanadium, is expensive and can only be obtained from China or Russia.
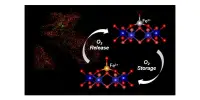
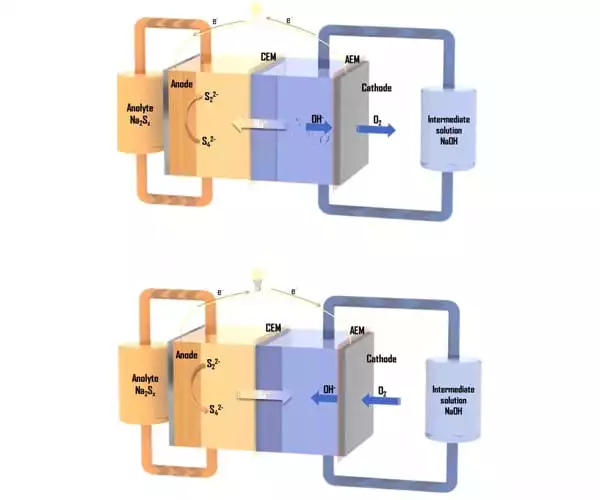
The Imperial team, coordinated by Professors Nigel Brandon and Anthony Kucernak, has been working on solutions that utilise lower-cost, commonly available materials. Their method employs a liquid as one electrolyte and gas as the other, in this case, polysulfide (sulphur dissolved in an alkaline solution) and air. However, the performance of polysulfide-air batteries is limited since no membrane can entirely allow the chemical reactions to take place while also preventing polysulfide from crossing over into the other side of the cell.
If the polysulfide crosses over into the airside, you lose material from one side, which decreases the reaction taking place there and inhibits the activity of the catalyst on the other. This decreases battery performance, thus that was a problem we had to tackle.
Dr. Mengzheng Ouyang
Dr. Mengzheng Ouyang of Imperial’s Department of Earth Science and Engineering explained: “If the polysulfide crosses over into the airside, you lose material from one side, which decreases the reaction taking place there and inhibits the activity of the catalyst on the other. This decreases battery performance, thus that was a problem we had to tackle.”
The researchers proposed using two membranes separated by a sodium hydroxide solution to separate the polysulfide and the air. The design’s advantage is that all materials, including the membranes, are reasonably cheap and readily available, and the design gives significantly more material options.
Hydrogen energy storage is another form of chemical energy storage in which electrical power is converted into hydrogen. This energy can then be released again by using the gas as fuel in a combustion engine or a fuel cell. Hydrogen can be produced from electricity by the electrolysis of water, a simple process that can be carried out with relatively high efficiency provided cheap power is available.
When compared with the best results obtained to date from a polysulfide-air redox flow battery, the new design was able to provide significantly more power, up to 5.8 milliwatts per centimetre squared.
Because cost is an important consideration for long-term and large-scale storage, the team also performed a cost study. They determined the energy cost (the price of the storage materials in relation to the amount of energy stored) to be roughly $2.5 per kilowatt hour.
The power cost (the rate of charge and discharge accomplished in relation to the price of the membranes and catalysts in the cell) was discovered to be roughly $1600 per kilowatt. This is now greater than would be viable for large-scale energy storage, but the team believes that future increases are easily achievable.
Nigel Brandon, Professor of Engineering and Dean of the Faculty of Engineering, stated: “Our dual-membrane technique is really intriguing since it offers up a plethora of new possibilities for this and other batteries. To make this cost-effective for large-scale storage, a relatively minor improvement in performance would be necessary, which might be accomplished through adjustments to the catalyst to increase its activity or through further improvements to the membranes utilised.”
Work in this area is already ongoing within the team, thanks to Professor Anthony Kucernak of the Department of Chemistry’s expertise in catalysts and Dr. Qilei Song of the Department of Chemical Engineering’s study into membrane technology.
If the improvements are made, the spin-off business RFC Power Ltd, which was founded to develop long-duration renewable energy storage based on the team’s research, plans to commercialize this novel design.