Unexpected chemicals generated by lithium and cesium under high pressure have been anticipated by researchers from Skoltech, Jiangsu Normal University, and other institutions. These novel materials exhibit unexpected chemistry, previously unseen crystal structures, and the highly desired trait of superconductivity, which results in the complete loss of electrical resistance below critical temperatures of roughly minus 223 to minus 213 degrees Celsius. The study came out in Nano Letters.
Conventional chemistry says lithium and cesium don’t form any compounds. However, it turns out that putting them under pressure does cause a number of chemicals to arise. A new study by a team of Chinese, Russian, and American scientists employed a more trustworthy algorithm and uncovered new and more stable phases, albeit some of them had been anticipated previously.
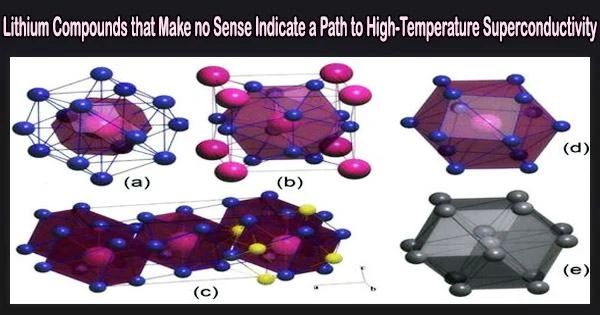
The researchers relied on fundamental theoretical principles and the USPEX crystal structure prediction algorithm, developed earlier by study co-author and Skoltech Professor Artem R. Oganov, to discover four lithium-rich compounds with seemingly bizarre formulas such as Li14Cs, which you won’t find in a chemistry textbook.
“Under normal conditions, if anything we would expect lithium to attract the electrons of cesium, which is the most electropositive element in the periodic table as most people know it: It is supposed to give up electrons, period,” Oganov commented.
Sure, from a technological standpoint, these critical temperatures are no good compared with what we’ve seen in polyhydrides the hydrogen-rich compounds of some metals. Yet this study deepens our understanding of lithium chemistry, and lithium as such could be interesting for superconductivity, perhaps in the form of a hypothetical ‘lithide’ compound so far we don’t know if it exists or how to spell it.
Professor Artem R. Oganov
A chemical element’s atom’s basic characteristic known as electropositivity describes how eagerly it accepts or, in the case of electronegativity, retains electrons. Together with his colleagues, Oganov shook up the periodic table by extending the notion of electronegativity into the realm of high pressures.
“Yet under pressure it is the other way around,” the researcher went on. “Cesium grabs the electrons of lithium, and this highly unusual chemical behavior leads to the formation of the four new compounds, two of which Li14Cs and Li6Cs turn out to have previously unseen crystal structure topologies.”
This is a fairly rare thing for compounds of just two elements. “Yet here we are, with two new topologies in one binary system,” Oganov added.
According to the team’s predictions, the four unique lithium and cesium compounds should be able to conduct electricity without experiencing any resistance.
So-called superconductors are substances that are sought after by scientists in the hope that they will one day enable power grids with unprecedented efficiency, ultrafast microchips, and electromagnets powerful enough to levitate trains or even control fusion reactors. This critical temperature ranges from roughly minus 223 to minus 213 degrees Celsius depending on the particular compound.
“Sure, from a technological standpoint, these critical temperatures are no good compared with what we’ve seen in polyhydrides the hydrogen-rich compounds of some metals. Yet this study deepens our understanding of lithium chemistry, and lithium as such could be interesting for superconductivity, perhaps in the form of a hypothetical ‘lithide’ compound so far we don’t know if it exists or how to spell it,” Oganov said, explaining that the lithium atom is very similar to that of hydrogen and could therefore stand in for it in a polyhydride-like compound.
Lithium, like hydrogen, is one of the lightest elements and has one valence electron, which is advantageous for superconductivity: It is well known that the critical temperature of an associated superconductor rises with decreasing atomic mass.
Like many other anomalies of high-pressure chemistry, the electronegativity inversion reported in this study namely, the Dong-Oganov reinvented electronegativity scale, published last year, predicted lithium giving up electrons to cesium.