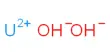
Limestone is a sedimentary rock type of carbonate; it is predominantly composed of calcium carbonate (CaCO3), typically in the form of calcite or aragonite. It forms most frequently in clear, wet, shallow marine waters. Dolomite, which contains a high percentage of the mineral dolomite, CaMg(CO3)2, is a closely related rock. Dolomite, a term now reserved for magnesium-deficient dolomites or magnesium-rich limestone’s, has been referred to as magnesium limestone. Limestone is typically an organic sedimentary rock that occurs from a shell, coral, algal, and fecal debris accumulation. It may contain large quantities of magnesium carbonate (dolomite) as well; clay, iron carbonate, feldspar, pyrite, and quartz are also commonly present in minor constituents.
By description, limestone is a rock containing at least 50 percent calcium carbonate by weight in the form of calcite. About 10% of the sedimentary rocks are lime. Karst landscapes are caused by the solubility of limestone in water and poor acid solutions, in which water erodes limestone for thousands to millions of years. In limestone bedrock, most cave systems are located. There’s a granular feel on most limestone. Their constituent grains vary in size from 0.001 mm to visible particles (0.00004 inches). The grains are small fragments of fossil animal shells in many instances.

(Limestones)
The shape of most limestone in shallow, calm, warm marine waters. That type of environment is where the required ingredients can be easily extracted from ocean water by organisms capable of forming calcium carbonate shells and skeletons. These species secrete shells made from aragonite or calcite and when they die, leave these shells behind. Ooids, peloids, and lime clasts are other carbonate grains that makeup limestone (intraclasts and extraclasts). Biological sedimentary rocks are limestones produced from this type of sediment. Their biological origin is also disclosed by the discovery of fossils in the soil.
Limestone, depending on the process of forming, can be crystalline, clastic, granular, or massive. Calcite, quartz, dolomite, or barite crystals may line tiny cavities in the rock. There are several limestone-forming habitats on Earth today. Most of them are located in areas of shallow water between latitudes of 30 degrees north and 30 degrees south. Limestone originates in the Caribbean Sea, the Indian Ocean, the Persian Gulf, the Gulf of Mexico, the islands of the Pacific Ocean, and the Indonesian archipelago. All of the limestone types are travertine, tufa, caliche, chalk, sparite, and micrite.
Travertine is a compact, banded variety of limestone formed along streams, particularly around hot or cold springs and where there are waterfalls. A limestone formed by evaporation at a hot spring, lakeshore, or other region is a rock known as “tufa”. Limestone, because of its rich fossil material, has long intrigued earth scientists. The study of fossils embedded in limestone and other carbonate rocks has derived a great deal of knowledge of the Earth’s chronology and creation. Calcium carbonate solubility (CaCO3) is primarily regulated by the partial water pressure of carbon dioxide (CO2). This is summed up in the answer:
CaCO3 + H2O + CO2 → Ca2+(aq) + 2 HCO3−

Limestone (Formation)
The calcium carbonate content of limestone gives it a property frequently used in rock identification; it effervesces 5% hydrochloric acid in contact with a cold solution. Limestone has important commercial value as well. The main source of raw materials for the fertilizer industry is limestone enriched in phosphate by the chemical action of ocean waters.
Limestone has various uses: as a building material, an integral concrete part (Portland cement), as a road base aggregate, as a white pigment or filler in products such as toothpaste or paints, as a chemical feedstock, as a soil conditioner, and as a common decorative addition to rock gardens for the production of lime. When heated to temperatures of 900 to 1,000 °C (1,650 to 1,800 °F), calcium carbonate will be dissociated from limestone, and carbon dioxide and lime will be formed, the latter having major applications in glass production and agriculture.
Information Sources: