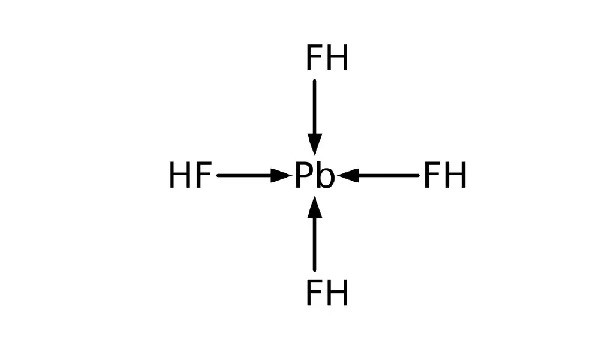
Lead tetrafluoride is a compound of lead and fluorine. The yellow solid (melting point 600 °C) is the only room-temperature stable tetrahalide of lead. It’s notable for its potential use in various applications, including materials science and chemistry. It is typically studied for its properties and behavior in different chemical environments.
Lead tetrafluoride is isostructural with tin(IV) fluoride and contains planar layers of octahedrally coordinated lead, where the octahedra share four corners and there are two terminal, unshared, fluorine atoms trans to one another.
Properties
- Chemical formula: PbF4
- Molar mass: 283.194 g/mol
- Appearance: white to beige crystals
- Density: 6.7 g/cm3
- Melting point: 600 °C (1,112 °F; 873 K)
Occurrences
- Natural Sources: It is not commonly found in nature as a standalone mineral. Instead, lead typically occurs in various lead ores, such as galena (PbS), where it is primarily extracted for use in lead compounds.
- Industrial Production: PbF₄ is synthesized in the laboratory and may be produced during the fluorination of lead compounds, particularly in the presence of fluorine gas or other fluorinating agents.
Applications
It is used in specialized applications such as in the production of other fluorides, in research settings, and potentially in electronic materials due to its unique properties.
Safety and Handling
Lead tetrafluoride should be handled with care, as lead compounds are toxic, and fluorides can be harmful. Proper safety precautions should be taken to minimize exposure.