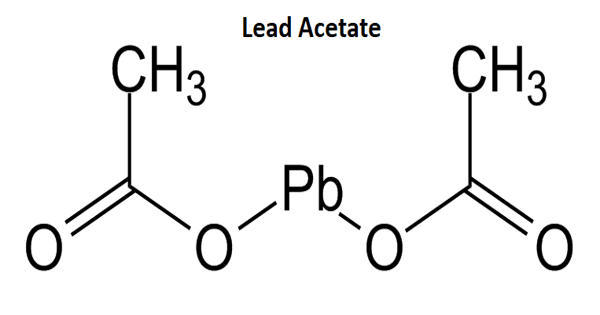
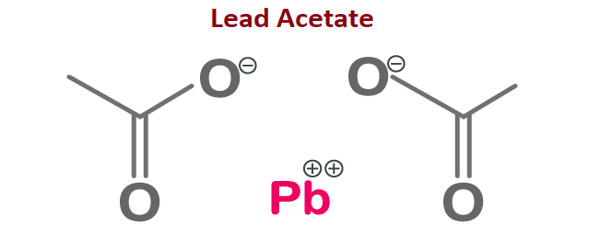
Lead acetate [Pb(CH3COO)2] is a white crystalline compound of lead with a sweetish taste. It is also known as lead acetate, lead diacetate, plumbous acetate, sugar of lead, lead sugar, salt of Saturn, or Goulard’s powder, is a white crystalline chemical compound with a slightly sweet taste. Known as the “sugar of lead”, it is water-soluble and one of the most bioavailable forms of lead. Like many other lead compounds, it is toxic. Similar to other lead compounds, it is very poisonous and soluble in water. It is used in making the white lead in medicines and as a mordant in dyeing.
Lead acetate was first produced in the United States in 1944. It is soluble in water and glycerin. It is stable under ordinary conditions of use and storage. With water it forms the trihydrate, Pb(CH3COO)2·3H2O, a colourless or white efflorescent monoclinic crystalline substance.
Lead acetate is a white crystal made by the action of acetic acid on litharge. Lead acetate is used in gold toning baths to modify the pH.
Properties
- Density: 3.25 g/cm³
- Molecular Weight/ Molar Mass: 325.29 g/mol
- Hydrogen Bond Acceptor: 4
- Melting Point: 280 °C
- Appearance: White to gray crystalline solid
- Solubility: Soluble in water
Production
Lead acetate can be made by boiling elemental lead in acetic acid and hydrogen peroxide. This method will also work with lead carbonate or lead oxide. The base acetate ion is found in both lead acetate and potassium acetate solutions; it is basic since its conjugate acid, acetic acid, is a weak acid.
Pb(s) + H2O2(aq) + 2 H+(aq) → Pb2 +(aq) + 2 H2O(l)
Pb2+(aq) + 2 CH3COO−(aq) → Pb(CH3COO)2(aq)
Lead acetate can also be made via a single displacement reaction between copper acetate and lead metal:
Cu(CH3COO)2 + Pb → Cu + Pb(CH3COO)2
Structure
The crystal structure of anhydrous lead acetate has been described as a 2D coordination polymer.
It is a white crystalline material with a sweet taste and is also classified by one of the following trivial names: lead sugar, lead sugar, Saturn salt and Goulard powder, respectively. In comparison, lead acetate trihydrate’s structure is a 1D coordination polymer. In the trihydrate, the Pb2+ ion’s coordination sphere consists of nine oxygen atoms belonging to three water molecules, two bidentate acetate groups and two bridging acetate groups. The coordination geometry at Pb is a monocapped square antiprism..
Uses
- The commercial form of lead acetate is used as a mordant in textile printing and dyeing, as a lead coating for metals, as a drier in paints, varnishes, and pigment inks, and as a colorant in hair dye.
- The substance is used as a reagent to make other lead compounds and as a fixative for some dyes.
- It is used as a water repellent, for mildew protection, and as a mordant for cotton dyes.
- In low concentrations, it is the principal active ingredient in progressive types of hair colouring dyes.
- Lead acetate is also used as a mordant in textile printing and dyeing, and as a drier in paints and varnishes. It was historically used as a sweetener in wines and in other foods and for cosmetics.
- It is also used in anti-fouling paints, waterproofing, insecticides, and the gold cyanidation process.
Information Source: