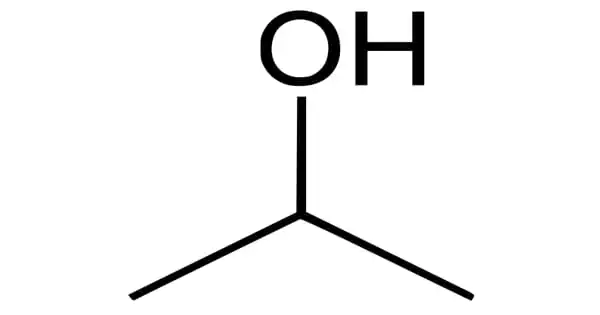
Isopropyl alcohol is a colorless, flammable chemical compound with a strong odor (chemical formula CH3CHOHCH3). It is one of the most common organic compounds in the alcohol family. The simplest example of secondary alcohol is an isopropyl group linked to a hydroxyl group, in which the alcohol carbon atom is attached to two other carbon atoms. It is a propyl alcohol isomer with antibacterial properties. It is a structural isomer of ethyl methyl ether and 1-propanol.
It is used in the production of a wide range of industrial and household chemicals, and it is a common ingredient in antiseptics, disinfectants, and detergents. It is a component of rubbing alcohol and is widely used as an organic solvent. It can also be abused for intoxication purposes.
Properties
Water, ethanol, and chloroform are all miscible with isopropyl alcohol. It is a colorless, volatile liquid with a strong musty odor similar to rubbing alcohol. It has a flash point of 53°F. It dissolves ethyl cellulose, polyvinyl butyral, as well as a wide range of oils, alkaloids, gums, and natural resins. Vapors are heavier than air and can cause minor irritation to the eyes, nose, and throat. Isopropyl alcohol, unlike ethanol or methanol, is not miscible with salt solutions and can be separated from aqueous solutions by adding a salt such as sodium chloride. The process, known colloquially as salting out, results in the separation of concentrated isopropyl alcohol into a distinct layer. It has a density of about 6.5 lb/gal.
Isopropyl alcohol reacts with water to form an azeotrope, resulting in a boiling point of 80.37 °C (176.67 °F) and a composition of 87.7 percent by mass (91 percent by volume) isopropyl alcohol. The melting points of alcohol mixtures are low. It has a slightly bitter taste and should not be consumed.
Isopropyl alcohol becomes increasingly viscous as temperature decreases and freezes at −89 °C (−128 °F). In the ultraviolet-visible spectrum, isopropyl alcohol has the highest absorbance at 205 nm.
Reactions
Acetone, the corresponding ketone, can be formed by oxidizing isopropyl alcohol. This can be accomplished through the use of oxidizing agents such as chromic acid, or through the dehydrogenation of isopropyl alcohol over a heated copper catalyst:
(CH3)2CHOH → (CH3)2CO + H2
In the Meerwein-Ponndorf-Verley reduction and other transfer hydrogenation reactions, isopropyl alcohol is frequently used as both a solvent and a hydride source. Using phosphorus tribromide, isopropyl alcohol can be converted to 2-bromopropane, or dehydrated to propene by heating with sulfuric acid.
Isopropyl alcohol, like most alcohols, reacts with active metals such as potassium to form alkoxides known as isopropoxides. The aluminum reaction (initiated by a trace of mercury) is used to prepare the catalyst aluminum isopropoxide.
Applications
As a rubbing-alcohol antiseptic, isopropyl alcohol is mixed with water. It’s also found in aftershave lotions, hand lotions, and other cosmetic products. It is used in industry as a low-cost solvent for cosmetics, drugs, shellacs, and gums, as well as to denaturize ethanol (ethyl alcohol). Cosmetics, skin and hair preparations, pharmaceuticals, perfumes, lacquer formulations, dye solutions, antifreezes, soaps, and window cleaners all contain it.