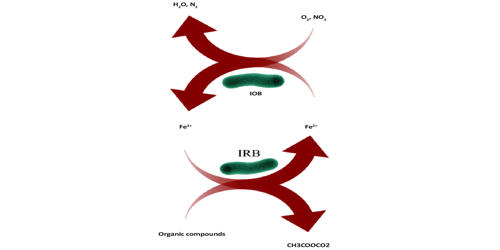
Iron-oxidizing bacteria are chemotrophic bacteria that derive the energy they need to live and multiply by oxidizing dissolved ferrous iron. These bacteria catalyze the oxidation of iron pyrite to ferric sulphate and sulphuric acid and increase the reaction rate against chemicals via. They are known to grow and proliferate in waters containing iron concentrations as low as 0.1 mg/L. However, at least 0.3 ppm of dissolved oxygen is needed to carry out oxidation. At the step of iron oxidation in such a system, a high density of cells of iron-oxidizing bacteria is essential for rapid iron oxidation.
Iron-oxidizing bacteria typically live in acidic, aerobic environments rich in both reduced-iron and -sulfur compounds; they grow poorly at pH values greater than 4. Iron is a very important element required by living organisms to carry out numerous metabolic reactions such as the formation of proteins involved in biochemical reactions, like Iron-sulfur proteins, Hemoglobin, and Coordination complexes. This element has a widespread distribution in the planet and is considered one of the most abundant in the Earth’s crust, soil, and sediments. Iron is one of the trace elements in marine environments. Its role in the metabolism of some chemolithotrophs is probably very ancient.
Biological iron oxidizers not only affect the cycling of iron but also efficiently minimize the concentrations of hazardous metals such as lead, nickel, copper, chromium, cadmium, and cobalt. The ferric iron generated after biological oxidation forms complexes with metals/metalloids present in their vicinity. Ferric ions produced by biological actions also act as a catalyst for oxidation of toxic metalloids such as arsenite (As III) converting it into a less toxic form. The ability of Fe-oxidizing bacteria to compete with the chemical oxidation of Fe(II) may depend on the rhizosphere pH. The kinetics of Fe oxidation are relatively slow at low pHs (<pH4) because Fe(II) is quite stable. Most importantly, bacterial iron oxidizers have commercially been employed in industrial bioleaching for the recovery of important elements and remediation of acid mine drainage water.
Some bacteria can use the oxidation of iron compounds as their primary energy source. Bacteria capable of using inorganic, rather than organic, molecules for their fueling reactions are termed chemolithotrophs, and iron-oxidizing bacteria are a major group in this nutritional category. Most importantly, bacterial iron oxidizers have commercially been employed in industrial bioleaching for the recovery of important elements and remediation of acid mine drainage water.