According to a recent study, researchers at Georgia State University’s Institute for Biomedical Sciences have created a novel oral medication for ulcerative colitis that aims to lessen inflammation in the gut flora.
The researchers used a two-step strategy in their study that was published in the journal Pharmaceutics to treat ulcerative colitis.
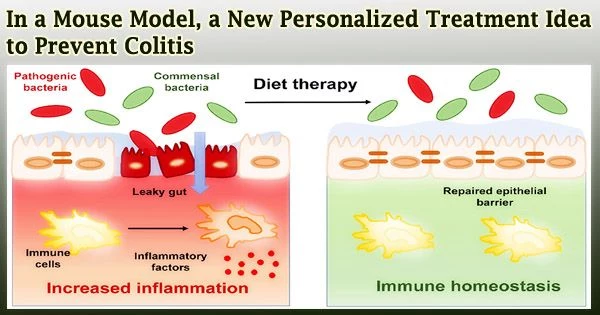
First, they used an anti-inflammatory medication candidate supplied via lipid nanoparticles to lessen inflammation in a mouse’s gut microbiome. A novel, efficient method to prevent ulcerative colitis was then developed by giving the same mouse the byproducts of these treated bacteria orally.
The results show that the nano formulation, M13/nLNP, changed the makeup of the inflamed microbiota toward that of the non-inflamed state. When the chemical profiles of secreted metabolites (the byproducts of metabolic reactions) were altered significantly by the altered microbiota composition, substantial protection against the development of chronic inflammation was produced in mice using these metabolites.
Over five million people worldwide suffer from the chronic inflammatory bowel disease (IBD) known as ulcerative colitis. According to studies, the development of ulcerative colitis is correlated with atypical gut microbiota composition.
It is effective to treat a number of chronic diseases, including ulcerative colitis, by changing the gut microbiota’s makeup. However, current approaches, including fecal microbiota transplants, require the transmission of organisms that are resistant to antibiotics, which increases the risk of catastrophic infections.
In this study, the researchers created an organism-free method in which the host’s gut microbiota were modified in test tubes, and then the metabolites they released were returned.
Our study demonstrates that modifying microbiota outside of the host using M13/nLNP effectively reshaped the microbial secreted metabolites. Oral transfer of these metabolites might be an effective and safe therapeutic approach for preventing chronic ulcerative colitis.
Dr. Didier Merlin
The researchers found that a natural lipid nanoparticle-encapsulated therapeutic candidate altered the makeup of inflamed gut microbiota that were cultivated outside of the host and the released metabolites by collecting feces from mice with chronic ulcerative colitis.
“Our study demonstrates that modifying microbiota outside of the host using M13/nLNP effectively reshaped the microbial secreted metabolites,” said Dr. Didier Merlin, a Distinguished University Professor in the Institute for Biomedical Sciences at Georgia State and a senior research career scientist at Atlanta Veterans Affairs Medical Center. “Oral transfer of these metabolites might be an effective and safe therapeutic approach for preventing chronic ulcerative colitis.”
The buildup of secreted metabolites may have an impact on how the microbiota’s composition changes, which is a study constraint. The released metabolites must be continually removed from the medium using a dynamic flowing device to ensure that the metabolites themselves do not effect how the drug formulation alters the composition of cultured microbiota.
Additionally, it is possible to further adjust other crucial elements including medication concentration, culture duration, and anaerobic gas composition.
“Our strategy to tackle the progression of ulcerative colitis might offer an alternative and complementary approach for better managing this disease,” said Dr. Chunhua Yang, a research assistant professor at the Institute for Biomedical Sciences at Georgia State.
“Although this study demonstrates the anti-inflammatory effects of metabolites modified outside of the organism, further investigations are required to characterize the specific bacteria that contribute to the anti-inflammatory metabolites and to identify anti-inflammatory metabolite structures.”
Co-authors of the study include Chunhua Yang (first author) and Didier Merlin of the Institute for Biomedical Sciences at Georgia State and the Atlanta Veterans Affairs Medical Center; Junsik Sung and Dingpei Long of the Institute for Biomedical Sciences at Georgia State; and Zahra Alghoul of the Institute for Biomedical Sciences and Department of Chemistry at Georgia State.
The National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health, the Department of Veterans Affairs and the Crohn’s and Colitis Foundation fund the study.