Molybdenum (Mo), chemical element, is a silvery-white metal that is ductile and highly resistant to corrosion, and its atomic number 42. It has one of the highest melting points of all pure elements only the elements tantalum and tungsten have higher melting points. Molybdenum is also a micronutrient essential for life. It is the 54th most common element in the Earth’s crust.
The name is from Neo-Latin molybdaenum, from Ancient Greek Μόλυβδος molybdos, meaning lead, since its ores were confused with lead ores. Molybdenum minerals have been known throughout history, but the element was discovered (in the sense of differentiating it as a new entity from the mineral salts of other metals) in 1778 by Carl Wilhelm Scheele. The metal was first isolated in 1781 by Peter Jacob Hjelm. It was often confused with graphite and lead ore. It has a high elastic modulus, and only tungsten and tantalum, of the more readily available metals, have higher melting points. Molybdenum has one of the highest melting points of all pure elements. Molybdenum is attacked slowly by acids.
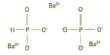
As a transistion metal, molybdenum easily forms compounds with other elements. Molybdenum comprises 1.2 parts per million (ppm) of the Earth’s crust by weight, but it is not found free in nature. The main molybdenum ore is molybdenite (molybdenum disulfide), but can also be found in wulfenite (lead molybdate) and powellite (calcium molybdate).
It is recovered as a by-product of copper or tungsten mining. Molybdenum is mined primarily in the United States, China, Chile, and Peru. World production is around 200,000 tons per year, according to the Royal Society of Chemistry (RSC).
Most molybdenum compounds have low solubility in water, but when molybdenum-bearing minerals contact oxygen and water, the resulting molybdate ion MoO2−4 is quite soluble. Industrially, molybdenum compounds (about 14% of world production of the element) are used in high-pressure and high-temperature applications as pigments and catalysts.
Facts –
- Atomic number (number of protons in the nucleus): 42
- Atomic symbol (on the periodic table of the elements): Mo
- Atomic weight (average mass of the atom): 95.96
- Density: 10.2 grams per cubic centimeter
- Phase at room temperature: Solid
- Melting point: 4,753 degrees Fahrenheit (2,623 degrees Celsius)
- Boiling point: 8,382 degrees F (4,639 degrees C)
The number of isotopes (atoms of the same element with a different number of neutrons): 24 whose half-lives are known with mass numbers from 86 to 110.
Most common isotopes: Mo-98 (24.1 percent); Mo-96 (16.7 percent); Mo-95 (15.9 percent); Mo-92 (14.8 percent); Mo-97 (9.6 percent); Mo-100 (9.6 percent); Mo-94 (9.2 percent).
History and Uses –
Molybdenite the principal ore from which molybdenum is now extracted was previously known as molybdena. Molybdena was confused with and often utilized as though it were graphite. Like graphite, molybdenite can be used to blacken a surface or as a solid lubricant. Even when molybdena was distinguishable from graphite, it was still confused with the common lead ore PbS (now called galena); the name comes from Ancient Greek Μόλυβδος molybdos, meaning lead. (The Greek word itself has been proposed as a loanword from Anatolian Luvian and Lydian languages).
The soft black mineral molybdenite (molybdenum sulfide, MoS2), looks very like graphite and was assumed to be a lead ore until 1778 when Carl Scheele analysed it and showed it was neither lead nor graphite, although he didn’t identify it. Others speculated that it contained a new element but it proved difficult to reduce it to a metal. It could be converted to an oxide which, when added to water, formed an acid we now know as molybdic acid, H2MoO4, but the metal itself remained elusive.
Scheele passed the problem over to Peter Jacob Hjelm. He ground molybdic acid and carbon together in linseed oil to form a paste, heated this to red heat in and produced molybdenum metal. The new element was announced in the autumn of 1781.
For the next century, molybdenum had no industrial use. It was relatively scarce, the pure metal was difficult to extract, and the necessary techniques of metallurgy were immature. Early molybdenum steel alloys showed great promise of increased hardness, but efforts to manufacture the alloys on a large scale were hampered with inconsistent results, a tendency toward brittleness, and re-crystallization. In 1906, William D. Coolidge filed a patent for rendering molybdenum ductile, leading to applications as a heating element for high-temperature furnaces and as a support for tungsten-filament light bulbs; oxide formation and degradation require that molybdenum be physically sealed or held in an inert gas. In 1913, Frank E. Elmore developed a froth flotation process to recover molybdenite from ores; flotation remains the primary isolation process.
During World War I, demand for molybdenum spiked; it was used both in armor plating and as a substitute for tungsten in high speed steels. Some British tanks were protected by 75 mm (3 in) manganese steel plating, but this proved to be ineffective. The manganese steel plates were replaced with much lighter 25 mm (1.0 in) molybdenum steel plates allowing for higher speed, greater maneuverability, and better protection.
Molybdenum is a valuable alloying agent, as it contributes to the hardenability and toughness of quenched and tempered steels. It also improves the strength of steel at high temperatures. Molybdenum is used in alloys, electrodes and catalysts. The Second World War German artillery piece called “Big Bertha” contains molybdenum as an essential component of its steel.
It is used in certain nickel-based alloys, such as the “Hastelloys(R)” which are heat-resistant and corrosion-resistant to chemical solutions. Molybdenum oxidizes at elevated temperatures. The metal has found recent application as electrodes for electrically heated glass furnaces and foreheaths. The metal is also used in nuclear energy applications and for missile and aircraft parts. Molybdenum is valuable as a catalyst in the refining of petroleum. It has found applications as a filament material in electronic and electrical applications. Molybdenum is an essential trace element in plant nutrition. Some lands are barren for lack of this element in the soil. Molybdenum sulfide is useful as a lubricant, especially at high temperatures where oils would decompose. Almost all ultra-high strength steels with minimum yield points up to 300,000 psi(lb/in.2) contain molybdenum in amounts from 0.25 to 8%.
Molybdenum-bearing enzymes are by far the most common bacterial catalysts for breaking the chemical bond in atmospheric molecular nitrogen in the process of biological nitrogen fixation. At least 50 molybdenum enzymes are now known in bacteria, plants, and animals, although only bacterial and cyanobacterial enzymes are involved in nitrogen fixation. These nitrogenases contain molybdenum in a form different from other molybdenum enzymes, which all contain fully oxidized molybdenum in a molybdenum cofactor. These various molybdenum cofactor enzymes are vital to the organisms, and molybdenum is an essential element for life in all higher eukaryote organisms, though not in all bacteria.
Molybdenum powders are used in circuit inks for circuit boards, and in microwaves devices and heat sinks for solid-state devices.
Information Sources: