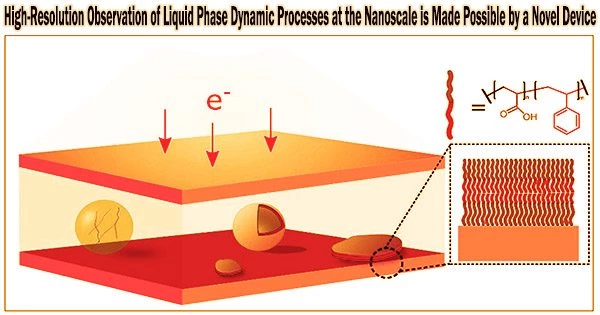
For the development of energy research, in-situ monitoring and recording of significant liquid-phase electrochemical reactions in energy devices is essential. A research team led by a scholar from City University of Hong Kong (CityU) recently developed a novel, tiny device to hold liquid specimens for transmission electron microscopy (TEM) observation, opening the door to directly visualizing and recording complex electrochemical reactions at nanoscale in real-time at high resolution.
The study team is confident that this cutting-edge technique will illuminate methods for creating a potent research instrument to solve the mysteries of electrochemical processes in the future.
Because the vacuum atmosphere in the chamber used to contain the specimens prevents the electrons from being absorbed or deflected along their routes and influencing observation, the use of traditional TEM is restricted to thin, stable, and solid samples.
Since liquid samples cannot be directly probed in a vacuum, they cannot be used in standard TEM. Fortunately, the development of the more sophisticated in-situ “liquid cell TEM” has made it possible to study liquid phase dynamic processes there. For example, one can watch crystal nucleation and growth in solution, electrochemical reactions in energy devices, and the biological functions of living cells.
A key element of TEM is the “liquid cell,” which holds the specimens for the electron beam to pass through, allowing for in-situ viewing. The incorporation of electrodes and the encapsulation of electrolytes in a compact “closed” liquid cell to prevent leakage and connect it to an external power source at the same time make high-quality liquid cells for TEM difficult to construct.
A research team co-led by Dr. Zeng Zhiyuan, Assistant Professor in the Department of Materials Science and Engineering at CityU, and Professor Li Ju from the Massachusetts Institute of Technology (MIT) successfully developed an efficient and novel method to fabricate “closed” electrochemical liquid cells, which can greatly improve the resolution of TEM with liquid samples.
“The newly developed closed liquid cell performs two main jobs: (1) enclosing the liquid samples in a closed container, thereby separating them from the microscope vacuum environment; and (2) confining the liquid samples to a thin enough liquid layer using two electron-transparent silicon nitride (SiNx) windows, so that electrons can travel through the liquid layer and image the reactions,” explained Dr. Zeng.
The electrochemical liquid cell designed by our customized nanofabrication method has thinner SiNx imaging windows (35nm) than commercial ones (50nm). It also has a thinner liquid layer (150nm) than that of commercial ones (1,000 nm). The thinner SiNx imaging windows and thinner liquid layer ensure that our fabricated liquid cell can capture electrochemical reactions with better TEM spatial resolution than commercial ones can.
Dr. Zeng Zhiyuan
The study team used cutting-edge nanofabrication techniques, including photolithography, to produce the essential part of in situ liquid TEM the liquid cell in order to create the high-performance, “closed” electrochemical liquid cells required for this approach. UV light is used in the photolithography process to transfer a geometric pattern from an optical mask to a substrate’s photoresist, a chemical that is light-sensitive.
The team independently created the bottom chip and the top chip, which were then combined. On the bottom chip, electrodes composed of titanium or gold were placed during the metal deposition process. The liquid cell was then filled with the electrolyte and shut.
The dynamic electrochemical responses of the liquid sample on the electrode surface can be captured in real time at high resolution using this unique liquid cell and transmission electron microscope thanks to the TEM operating system’s integration of a high spatiotemporal resolution camera.
“The electrochemical liquid cell designed by our customized nanofabrication method has thinner SiNx imaging windows (35nm) than commercial ones (50nm),” explained Dr. Zeng. “It also has a thinner liquid layer (150nm) than that of commercial ones (1,000 nm). The thinner SiNx imaging windows and thinner liquid layer ensure that our fabricated liquid cell can capture electrochemical reactions with better TEM spatial resolution than commercial ones can.”
The research team predicts that the development of the electrochemical liquid cell, with the choice of patterned metal electrodes and the encapsulated liquid electrolytes in the liquid cell, will lead to a significant increase in opportunities and applications for the in-situ TEM observation of electrochemical reactions.
Beyond TEM, this newly suggested fabrication approach can be used to additional in-situ techniques. For example, a proper adjustment to this protocol would be suitable for the fabrication of electrochemical liquid cells for in-situ X-ray characterizations of electrochemical reactions (X-ray absorption spectroscopy, X-ray diffraction, etc.).