According to a University of Bristol-led study of more than 8 million patients, high doses of a commonly used drug used in the hormonal treatment of conditions like excessive hair growth, early puberty, and prostate cancer are linked to an increased risk of meningioma, the most common type of benign brain tumor.
The study is published in Scientific Reports today (Friday 4 Feb 2022).
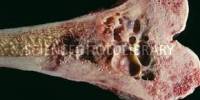
Meningiomas are benign tumors that typically develop slowly. However, because they can compress nearby brain tissue, nerves, and blood vessels, as well as induce pressure effects inside a fixed cranial vault, they can be very disabling.
a mass of tissue that develops abnormally when cells do not die on schedule or expand and divide more often than they should. Cancer-free tumors might be benign or malignant (cancer). Although benign tumors can become enormous, they do not penetrate or spread to surrounding tissues or to other areas of the body.
Malignant tumors have the potential to invade or spread to neighboring tissues. The lymphatic and circulatory systems of the body can also distribute them to other areas of the body. also known as a tumor. According to how quickly they grow and how likely it is that they will return following therapy, brain tumors are graded.
Tumors in grades 1 and 2 are low grade, whereas those in grades 3 and 4 are high grade. Meningiomas have been linked to hormone therapies, particularly long-term, high-dose use of the medication cyproterone acetate (CPA), according to recent investigations.
However, our study underscores the current limited evidence about the risk of intracranial meningioma associated with low dose cyproterone acetate. It is still unknown whether or not cyproterone acetate below a certain threshold may be completely safe in terms of the risk of meningioma. The results obtained herein suggest the necessity for further clinical research on intracranial meningioma associated with cyproterone acetate.
Keng Siang Lee
Male patients with incurable prostate cancer who also require hormonal therapy for male-to-female transsexuals or hirsutism typically receive high dosages of cyproterone acetate (> 50 mg/day).
The medication is frequently used with oestradiol at lower levels (2–10 mg/day) to treat female seborrhea and androgen-associated alopecia. Patients with these tumors had historically had very poor prognoses, with typical survival times of only a few weeks.
More advanced diagnostic methods, together with cutting-edge surgery and radiation techniques, have increased survival rates by up to years and improved patients’ quality of life after diagnosis.
To evaluate the evidence of a link between cyproterone acetate and incidence of meningiomas given the drug’s widespread use, researchers from the Universities of Bristol, Cambridge, and the National University of Singapore conducted a systematic review and meta-analysis study using four studies and a sample of 8,132,348 patients.
165,988 patients who were taking cyproterone acetate at various dose levels were included in the sample. Using this information, the team examined the incidence of meningioma in patients receiving high vs low doses of cyproterone acetate and discovered a statistically significant link between the use of the high dose and a higher risk of developing meningioma. Low doses, though, did not show this connection.
Keng Siang Lee, a medical student and the study’s lead author from Bristol Medical School at the University of Bristol, said:
“The cause of meningiomas is controversial but there is strong evidence to suggest a plausible role for sex hormones in the onset of meningioma. We know it has a predilection for females especially after puberty. Furthermore, fluctuations in meningioma growth during the menstrual cycle, pregnancy, and breastfeeding have also been well-documented. We are also aware of the well-characterised distribution of progesterone, oestrogen, and androgen receptors in certain meningiomas located at the base of the skull.”
“In light of these results, prescription of high-dose cyproterone acetate, especially for off label indications, should be considered carefully. Additionally, we suggest that routine screening and meningioma surveillance by brain MRI offered to patients prescribed with cyproterone acetate is likely a reasonable clinical consideration if given at high doses for long periods of time.”
“However, our study underscores the current limited evidence about the risk of intracranial meningioma associated with low dose cyproterone acetate. It is still unknown whether or not cyproterone acetate below a certain threshold may be completely safe in terms of the risk of meningioma. The results obtained herein suggest the necessity for further clinical research on intracranial meningioma associated with cyproterone acetate.”